nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Electrosynthesis of nitrates from urine
There seem to be lots of threads dealing with the synthesis of chlorates and perchlorates by electrolysis, though no topic exists about
electrosynthesis of nitrates from urea or ammonia.
Most articles concerning electrolytic ammonia oxidation seem to be aimed at nitrogen removal from urine as N2(g) and describe low yields (11%) of
nitrates (based on ammonia) using a simple graphite/graphite based anode/cathode cell (https://doi.org/10.1016/j.watres.2014.11.031). Overall, it seems difficult to avoid conversion to N2(g) perhaps due to some sensitive
hydroxylamine intermediate (soluble transition metals?) or lower barrier for cathodic reduction of nitrates as compared to chlorates. Relative high
nitrate production rates and yields seem feasible though: https://doi.org/10.1039/C7EW00014F
Another review on electrolytic ammonia oxidation mentions: "In neutral solutions (also at high anodic potentials), urea was decomposed mainly to
nitrite and nitrate ions and resulted in CO2 generation" Referrencing to a Russian article that I could not find online:
Osetrova, N. V., and A. M. Skundin. "Anodic oxidation of urea in neutral solutions." Russian journal of electrochemistry 30.10 (1994):
1145-1147.
Would anyone be able to download this one and add it to SMDB?  Urea nitrate may
also be isolated relatively easily from these solutions due to relative low solubility in cold water. Urea nitrate may
also be isolated relatively easily from these solutions due to relative low solubility in cold water.
Any thoughts whether this would be efficiently feasible and if yes, what would be the best conditions for high nitrate conversion? Overall, a near
neutral pH seems best for nitrate synthesis, but overall it seems only small concentrations of nitrate can be realized before cathodic reduction
becomes significant. From the articles referenced above it also seems an interesting question what the role of chloride is in ammonia oxidation. Also
read something about lead/silver or nickel based anodes being especially advantageous, refs will follow. Would be cool if we could get this to work,
especially seen the importance of nitrates/nitration in amateur chemistry. Preventing cathodic reduction somehow or precipitating the nitrates (as a
quaternary ammonium salt maybe?) could help.
Instead of urea, an ammonium (bi)carbonate solution would also seem convenient (might be more conductive as solution)...blow CO2 through ammonia until
pH is only slightly alkaline --> perform electrolysis using a porous graphite anode and small surface graphite cathode at 10-30 mA/cm2 -->
distill off ammonium carbonate for recycling...any nitrate should be left as ammonium nitrate...Doable? 
[Edited on 1-12-2020 by nitro-genes]
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
You need sodium perchlorate catalyst for this process and also membrane cell and PbO2 electrode membrane for preventing nitrate reduction at cathode
and PbO2 is the only electrode capable of surviving nitric acid and ozone with perchlorate
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Hi mysteriusbhoice, why do you suggest PbO2 anode? Do you think high oxygen overpotential of the anode is important? Are you aware of any references
using PbO2 anodes for electrolysis of ammonia/urea/amines? Not sure it would survive or not be inactivated during electrolysis.  I'm pretty sure to have seen mention of lead/2% silver anodes for nitrate
electrosynthesis somewhere, can't find the reference again... A membrane would prevent cathodic reduction I guess, though indeed, the pH would
decrease probably for the anolyte. Also not sure if such low pH would still allow anodic oxidation to nitrates. I'm pretty sure to have seen mention of lead/2% silver anodes for nitrate
electrosynthesis somewhere, can't find the reference again... A membrane would prevent cathodic reduction I guess, though indeed, the pH would
decrease probably for the anolyte. Also not sure if such low pH would still allow anodic oxidation to nitrates.
Here a couple of relevant articles it seems.
Article 2 seems interesting...again they mention that electrolysis of neutral sulfate or phosphate buffered solutions of urea using platinum
electrodes and high current densities results in selective conversion to nitrate and nitrite with almost 90% current efficiency. Also encouraging
seems that even with very small urea concentrations anodic oxygen evolution does not seem to be significant. Figure 2 seems to suggest that the
nitrate selectivity only applies to dilute solutions unfortunately, with increasing urea and/or nitrate concentrations oxidation to N2 becomes
increasingly more significant, pointing towards cathodic reduction or other detrimental reactions, such as polymerization of the urea (described
better in part 1: Osetrova, N. V., and A. M. Skundin. "Anodic oxidation of urea in neutral solutions." Russian journal of electrochemistry 30.10
(1994): 1145-1147.)
Article 3 looked at the effect of temperature and NaF vs NaCl additions on the efficiency of nitrate conversions. Overall, the presence of chloride in
these setups seemed to increase oxidation to N2(g) relative to nitrate, meaning direct conversion of urine would probably be less efficient.
Interestingly, NaF seems very efficient over a large urea concentration range, though nothing is stated about effect of total amp.h delivered, pH of
electrolyte or nitrate concentrations involved. I would expect aqueous NaF to behave relatively inert during electrolysis (similar to sulfates), but
maybe there is some interaction with the platinum electrodes?
Still have to let it sink in somewhat, though nitrate synthesis by electrolysis seems possible at least! 
Attachment: Anodic oxidation urea russian 2.pdf (39kB)
This file has been downloaded 474 times
Attachment: Anodic oxidation urea russian 3.pdf (24kB)
This file has been downloaded 470 times
[Edited on 2-12-2020 by nitro-genes]
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
som1 told me to try using cyanuric acid instead of urea for anodic oxidation
https://www.sciencemadness.org/whisper/viewthread.php?tid=15...
this thread talks about using melamine and cyanuric acid is that without the amines but still has the amides.
maybe mix of cyanuric acid and ammonia salts??
https://pubs.acs.org/doi/10.1021/es102423u#:~:text=The%20rea...
there is also that thread which talks about the chlorination of urea using Cl2 gas and I have tried chlorate electrolyte instead of NaCl and it
produced an acidic solution which eats copper but its incredibly dillute.
Now I think PbO2 and perchlorate electrolyte might help as a good catalyst since perchlorate electrolyte can emit ozone and perhaps help aid the
oxidation of urea hopefully not into N2.
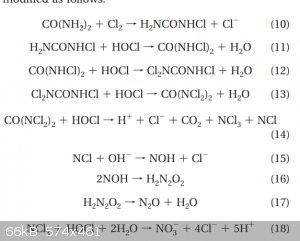
I wanted to continue doing with chlorate however I notice a precipitate form that seemed to be unstable when collected it was NH4ClO3 and I was like
NOOOPE.
The chlorination of urea produces NCl3 and at high temps it hydrolyzes into NO2 and that makes HNO3.
Ive also snapped a screenshot of some interesting reaction scheme though I lost the source paper on it.
[Edited on 6-12-2020 by mysteriusbhoice]
|
|
|
Mister Double U
Harmless

Posts: 20
Registered: 21-2-2025
Member Is Offline
|
|
Hello fellow mad scientists!
I am new to the forum as a registered user (have been browsing around here for ~15yrs though).
I want to dedicate my first post to the anodic oxidation of urea/ammonia to nitrate.
Nitrates have been hard to acquire for the hobby chemist in many countries for some time now and are essential for projects like metal refining and
making compounds in oxidative melts.
I have been looking at the topic for a while now and tried:
- Oxidation in strongly basic environment with copper catalyst -> Semi successful, not very reproducible.
- Oxidation in strongly acidic environment with silver catalyst -> Successful
- Oxidation in ~neutral electrolyte without catalyst -> Successful
Today I am reporting on my last attempt in neutral pH environment.
[Edited on 22-2-2025 by Mister Double U]
|
|
|
Mister Double U
Harmless

Posts: 20
Registered: 21-2-2025
Member Is Offline
|
|
Experiment:
1. 125g of NaHCO3 are added to a 500ml beaker and filled up with distilled hot water to 500ml. Not all of the salt dissolves.
2. A stir bar is inserted into the beaker and set to lowest rpm.
3. The electrode setup is inserted into the liquid and current is turned on:
I = 3A
Electrode Surface: ~8cm^2
Electrode Distance: 4mm
Kathode Material: Perforated Nickel (a little pitted from a prior nickel-plating attempt)
Anode Material: Boron Doped Diamond on silicon substrate (left electrode - not in its best shape anymore after being once used in strong alkaline
electrolyte for ~2 days)

Picture of cell after it had already been emptied:

4. Current is run through the cell without addition of any urea for half a day to destroy eventually present chloride ions (oxidation to perchlorate).
5. During regular electrolysis 4g of urea are added twice per day in the form of 12ml diesel exhaust fluid. The initial undissolved NaHCO3 dissolved
in the first 3 days of the electrolysis.
6. Electrolysis was run for 10.5 days.
7. After stopping the current the electrolyte is neutralized with 190g 50% sulfuric acid. CO2 evolution stopped after 65g.
8. Solution was evaporated in a round bottom flask until most of the water was gone.
9. After the flask had cooled down an additional 150g of concentrated sulfuric acid have been added and distillation was conducted until no more
liquid came over.


Results:
129g of liquid with density 1.234 g/cm^3 were collected.
This density corresponds to a 37.77% solution of HNO3 in H2O.
As a result, 48.7g of HNO3 were created.
This corresponds to 21.9% current efficiency.
[Edited on 22-2-2025 by Mister Double U]
|
|
|
Mister Double U
Harmless

Posts: 20
Registered: 21-2-2025
Member Is Offline
|
|
Discussion:
My initial idea for this experiment came from 'Anodic oxidation urea russian 2' paper where it is stated that urea to nitrate conversion can reach up
to 80% current efficiency when urea concentration is very low.
This was the reason for the daily addition of urea. In other words, it was as much added as the cell could oxidize to nitrate. The problem here is
that some of the nitrate gets reduced back to ammonia which increases the concentration of nitrogen in its -3 oxidation state and this decreases the
current efficiency towards the nitrate formation on the anode.
To better understand the whole picture, I wrote a program in R language to model the behaviors of the different concentrations. All -3 nitrogen was
calculated as ammonia under the hood of the program.
Here the results of the code with parameters tweaked to the experiment:

Assumed were 3 reactions:
Anode: NH3 -> NO3
Anode: NH3 -> N2
Cathode: NO3 -> NH3 (reaction depends on current density & nitrate concentration)
The program allows for input of process variables:

Here the link to the program, if someone wants to play around with this (Installation of R & RStudio is required prior to running it):
Attachment: Concentrations.zip (4kB)
This file has been downloaded 40 times
Double-click the 'Source' icon in the folder to load it in RStudio and then click the little arrow next to the Source button and select 'Source with
Echo'

The window for the process variables should pop up now.
Heads up: The pop-up window for the parameters is sometimes hidden behind the program window.
Once the program ran (time varies based on selections) a graph with the concentration profiles will be created on the right side of the program
window.
Y'all have fun :-)
[Edited on 22-2-2025 by Mister Double U]
[Edited on 22-2-2025 by Mister Double U]
|
|
|
Mister Double U
Harmless

Posts: 20
Registered: 21-2-2025
Member Is Offline
|
|
Experiment with cathodic Reduction Inhibitor:
Ok, since the current efficiency of the experiment above seems a little discouraging, I want to add a silver lining.
Here the quick writeup of an experiment I did a while ago with a similar setup, but Potassium Dichromate added to inhibit the reduction reaction of
nitrate:
1 g/l Potassium Dichromate
250g NaHCO3 in 1 liter H2O
Addition of urea 2x 4g per day (2x 12ml diesel exhaust fluid)
Current = 3A
Cathode: Titanium mesh
Anode: BDD (same as above)
Electrode surface: 8cm^2
Electrode distance: 5mm
Required Voltage: 6.3V
Vessel: 1l beaker with stirring on low
Duration: 12days
Result after distillation with H2SO4: 146g HNO3
Current efficiency: 57.5%
So, a little better with K2Cr2O7. For small scale/hobby nitrate production definitely a viable route.
Last comment: At some time, I also tried platinum plated titanium mesh as anode and this definitely made nitrate, but the electrode gave up after ~2
weeks.
Ok, I guess I am done posting for today :-D
|
|
|
Mister Double U
Harmless

Posts: 20
Registered: 21-2-2025
Member Is Offline
|
|
Another Run
Here the results of another run which just finished:
0.5g K2Cr2O7
100g NaHCO3 in 0.5 liter H2O
Addition of urea 2x 4g per day (2x 12ml diesel exhaust fluid)
Current = 3A
Cathode: Nickel (perforated)
Anode: BDD (same as above)
Electrode surface: 8cm^2
Electrode distance: 4mm
Required Voltage: 6.3V
Vessel: 0.5l beaker with stirring on low
Duration: 5days
Cell Temperature: 50-60C (estimated by touch)
Cell at Startup:

Cell when finished:

Cell content after evaporation of some of the Water:

After Addition of 200g Sulfuric Acid (excess to get all NO3 out):

Flask after Destillation:

Results:
122g of liquid with density 1.33g/cm^3 were collected.
This corresponds to 54% HNO3 concentration.
Calculated through interpolation from table: (1.33-1.31)/(1.37-1.31) = (X-50)/(60-50)

HNO3 produced: 66.2[g]
HNO3 theoretical: (5[days] * 24[hrs] * 3600[s] * 3[A]) / (96485[C/mol] * 8[electrons]) * 63 [g/mol] = 105.77[g]
Current Efficiency: 66.2[g] / 105.77[g] = 62.5%
Side notes:
1. At the end of the run some cell liquor was dropped into hydrochloric acid and no CO2 evolution was visible. That means that all the NaHCO3 got
converted to Nitrate.
2. After distillation all the Dichromate seems to have been reduced to Cr(III).
Best Greetings!
[Edited on 3-3-2025 by Mister Double U]
|
|
|
Mister Double U
Harmless

Posts: 20
Registered: 21-2-2025
Member Is Offline
|
|
Test: Does MMO create Nitrate in a Carbonate Electrolyte -> No
Hello Folks,
Last week I ran the same cell as above with an MMO anode.
Unfortunately, I do not know the exact type - I bought it of Ebay many years ago. It is stable towards Oxygen generation though, which I found out
through experimenting with it.
Result -> No nitrate was generated at all.
0.5g K2Cr2O7
100g NaHCO3 in 0.5 liter H2O
Addition of urea 2x 5.3g per day (2x 16ml diesel exhaust fluid)
Current = 4A
Cathode: Copper Spiral
Anode: MMO
Electrode surface: 50cm^2
Electrode distance: ~2cm (average for a plate anode sitting in a copper spiral)
Required Voltage: ~5V (did not exactly measure only read from the dial of my variac)
Vessel: 0.5l beaker with stirring on low
Duration: 6.5days
Cell Temperature: 30C (estimated by touch)
So, it looks that at least some types of MMO do not generate any nitrate in a reaction like this. Which exact effect the much lower current density on
the anode had on this I cannot tell. I would think though, that at least a little nitrate would have been created if this anode was capable of doing
so (gut feeling).
However, I have used such an anode in the past for the acidic, silver catalyzed route of ammonium oxidation to nitrate with success.
If anyone is interested in that procedure, I could see if I can replicate my experiment from back then.
At the moment it seems though, that there is not much interest in this topic. So, I'll stop for now.
|
|
|