| Pages:
1
2
3
4
5
..
7 |
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
It is not down, you just may have been going to his old site.
The most recent one is: http://www.cdodgyd.f2s.com/perchloric.htm
|
|
|
Blaster
Hazard to Self
 
Posts: 54
Registered: 7-11-2003
Location: UK
Member Is Offline
Mood: perchloric!
|
|
Quite right Rogue Chemist! I will ensure my web page is always there.
I've just logged on for the first time in months - glad to see the thread is still going strong!
If you want to contact me, you could always post me a message on this board.
|
|
|
Nerro
National Hazard
   
Posts: 596
Registered: 29-9-2004
Location: Netherlands
Member Is Offline
Mood: Whatever...
|
|
Could alkylperchlorates be(somewhat) stabilsed by making the alkylpart longer?
#261501 +(11351)- [X]
the \"bishop\" came to our church today
he was a fucken impostor
never once moved diagonally
courtesy of bash
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
Conversely... perchloroacetylene, anyone? 
No, seriously. Chloroacetylene reacted with a perchlorate (preferably lead or silver perchlorate, the resulting halide is insoluble, thus shifting the
equilibrium to the right) would very likely give you HCC(ClO4), which has perfect OB and is probably quite unstable as well. The one thing that bugs
me about organic perchlorates is the C-O bond, which is why I can't consider perchloroacetylene a perfect explosive. The oxygen atom is half
reduced, and the carbon atom is one quarter oxidized, it's like having a quarter of a CO2 molecule pre-inserted, a dead ballast. If one inserts a
nitrogen atom between HCC and ClO4 and then attaches an azide group to the atom, then perfect OB will be retained and there will be no more dead
ballast. HCCN(N3)ClO4 is something I can call a perfect explosive.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
About chlorine(VII) compounds, all of which are either perchloric acid HClO4, perchlorates ClO4-, the heptoxide Cl207,and peroxide ClO4•, and the
cation ClF6+, and possibly some oxyfluorides like CLO3F: does anyone know if someone has had any success in obtaining argon(VIII) or other argon
compounds by the radioactive decay of Cl-36 (or other chlorine radioisotopes with an excess of neutrons) in the form of such compounds? BromicAcid
might know.
|
|
|
Blaster
Hazard to Self
 
Posts: 54
Registered: 7-11-2003
Location: UK
Member Is Offline
Mood: perchloric!
|
|
In answer to Nerro's question, the answer is yes!
The ethyl ester is slightly less sensitive than the methyl for a start. If you have a look at the bottom of my webpage you will see that the glycol
monoperchlorates are very much more stable than the simple esters.
Any electron donation helps stabilise the incredibly weak CO-ClO3 bond.
[Edited on 29-1-2005 by Blaster]
|
|
|
Chris The Great
Hazard to Others
  
Posts: 463
Registered: 29-10-2004
Location: Canada
Member Is Offline
Mood: No Mood
|
|
Hmmm, would a C-CLO3 bond be stronger? If so it might be interesting, however I have a feeling it may be more unstable, or harder to synthesis
because chloric acid, which is very unstable IIRC, will be needed. And heating super-unstable explosive acids is generally not a good idea,
especially when they are powerful oxidizers mixed with some fuel as well.
Of course, I assume there is some vastly obvious reason why this wouldn't work anyway 
|
|
|
Nerro
National Hazard
   
Posts: 596
Registered: 29-9-2004
Location: Netherlands
Member Is Offline
Mood: Whatever...
|
|
1) You do not need Chloric acid for that synthesis. Did you see any perchloric acid used in the synth of EtClO4? You could use Ba(ClO3)2.
2) A C-ClO3 bond does not exist for as far as I know. The Cl is pentavalent in this ion which means one oxygen atom will be left with a free electron
dangling about. The bond would be accordingly: C-O-ClO2.
| Quote: | In answer to Nerro's question, the answer is yes!
The ethyl ester is slightly less sensitive than the methyl for a start. If you have a look at the bottom of my webpage you will see that the glycol
monoperchlorates are very much more stable than the simple esters.
Any electron donation helps stabilise the incredibly weak CO-ClO3 bond. |
That was exáctly
what I thought! The Alkylgroup will quite readilly "donate" it's electrons to the rather stressed Cl atom (for as far as I understand
this stuff). Perhaps pentanole can be reacted with H2SO4 to Pe-O-SO3H (Pe = pentyl) so that the bariumsalt of that can be made.
A question about the synthesis, could CaCO3 be used rather than BaCO3? It seems to me that as long as the salt of the sulfate is poorly soluble any
cation could be used.
[Edited on 29/1/2005 by Nerro]
[Edited on 29/1/2005 by Nerro]
#261501 +(11351)- [X]
the \"bishop\" came to our church today
he was a fucken impostor
never once moved diagonally
courtesy of bash
|
|
|
Blaster
Hazard to Self
 
Posts: 54
Registered: 7-11-2003
Location: UK
Member Is Offline
Mood: perchloric!
|
|
Quote:
"Perhaps pentanole can be reacted with H2SO4 to Pe-O-SO3H (Pe = pentyl) so that the bariumsalt of that can be made.
A question about the synthesis, could CaCO3 be used rather than BaCO3? It seems to me that as long as the salt of the sulfate is poorly soluble any
cation could be used."
I see no reason why the pentyl ester couldn't be made using the same technique, although Meyer and Spormann began to have difficulties with the
propyl ester because extra heat is required to distill it and that is not a good idea with these compounds!
As to whether Ca could replace Ba, I don't know. I suspect not for thermodynamic reasons. If someone wishes to calculate the bond energies etc
then feel free, but the basic driving force for the reaction is the formation of the very stable BaSO4.
Incidently, BaEtSO4 IS soluble. Drying it takes some doing!
[Edited on 29-1-2005 by Blaster]
|
|
|
Nerro
National Hazard
   
Posts: 596
Registered: 29-9-2004
Location: Netherlands
Member Is Offline
Mood: Whatever...
|
|
Low pressure distillation seems like an idea. As long as the T can be kept below 40 degrees C it should be fine.
I remember reading a reference to the use of KEtSO4 in a similar synthesis so perhaps the switch can be made. I'm not sure.
#261501 +(11351)- [X]
the \"bishop\" came to our church today
he was a fucken impostor
never once moved diagonally
courtesy of bash
|
|
|
BromicAcid
International Hazard
    
Posts: 3253
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Did anyone look at the images that I scanned in? It's on the preparation of longer chain perchlorates under very easy to duplicate circumstances
(comparatively easy). By reacting an unsaturated hydrocarbon with a slurry of a perchlorate salt in sulfuric acid with an inert hydrocarbon layer
above it, the perchlorate goes into the hydrocarbon layer and is easily extracted, distillation avoided and so is the complication of making barium
ethyl sulfate and others.
Also, Chris, I was at one time thinking about organic chlorates, specifically their manufacture by reacting heated basified alcohol with chlorine
analogous to the process to make organic hypochlorites. However considering how stable I would assume them to be I won't be the one to try this,
and the increase in temperature would most likely just lead to chlorination of the molecule such as the preparation of chloral hydrate from ethanol
and the preparation of chloroform from ethanol. And John WW, I can't say I've heard of making argon compounds by decomposition of
radioactive chlorine compounds, although I feel compelled to try and find out more.
|
|
|
Chris The Great
Hazard to Others
  
Posts: 463
Registered: 29-10-2004
Location: Canada
Member Is Offline
Mood: No Mood
|
|
Yeah, I figure they would be likely to just explode sudden without any reason upon forming (or, with my luck they would form in large amounts and then
collect together in a puddle and THEN explode.).
Nerro, I forgot that barium perchlorate was used, I read through the thread quickly the night before and for some reason though that perchloric acid
was used.. 
My bad.
If it was attempted to make an organic chlorate, I would want to keep the reaction temperature as low as possible, hence the acid might work
better.....
|
|
|
BromicAcid
International Hazard
    
Posts: 3253
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Nerro, you mentioned ethyl perbromate, something I would have never thought I would find a reference for, then today, lo and behold:
JACS 97:2 Jan 22, 1973 pp 267
Experimental preparation of isopropyl perbromate. Ethyl perbromate and others were also attempted, but the method, reacting an alkyl bromide with
silver perbromate, gave unsatisfactory yields with other alkyl groups.
A little off topic but these quotes outline the preparation of silver perbromate, whose preparation could easily be modified to make silver
perchlorate (the simple reaction of silver oxide with perchloric or perbromic acid is normally complicated by the EXTREME hygroscopicity of the
product [when attempting to dry over concentrated sulfuric acid it actually GAINS weight]) Also the preparation of isopropyl perbromate is of some
intrest. | Quote: | Silver Perbromate. Silver oxide (5.80 g, 0.025 mol) was added in portions with stirring, to 100 ml of 0.5 M perbromic acid. The
mixture was stirred for 2 hr at ambient temperature and was then filtered. The bulk of the water was removed from the filtrate under vacuum, and 100
ml of benzene was added. The remaining water was removed by azeotropic distillation using a Dean-Stark trap. The resulting benzene solution was
filtrered hot. When the solution was cooled to room temperature, 100 ml of hexane was added. The solvent was decanted from the precipitated salt,
and the salt was dried briefly at 25C (0.05 mm) and was then heated with a 70C bath at 0.05 mm for 6 hr to remove absorbed solvent. The product, 11.1
g (88%), was a white, hygroscopic, crystaline solid.
.............
Isopropyl Perbromate. A solution of 0.123 g (1.0 mmol) of isopropyl bromide in 1 ml of carbon tetrachloride was added dropwise, with
stirring, to a suspension of 0.252 g (1.0 mmol) of silver perbromate in 4 ml carbon tetrachloride, maintained at -20C by means of a carbon
tetrachloride - Dry ice slush bath. The reaction mixture was kept at -20C for 15 min. The silver bromide was removed by filtration, giving a pale
yellow solution.
.............
The yield of isopropyl perbromate was 95% determined by nmr integration using chlorobenzene as a quantitative internal standard. The only impurity
detected was 1% of acetone. The decomposition of isopropyl perbromate solutions was monitored similarly by nmr. The solutions showed no
decomposition within several hours at -20C. At ambient temperature, the compound decomposed with a half-life of about 30 min to give acetone as the
only product detectable by nmr. The yield of acetone was 90% in 24 hr. The solution became red-orange in color. |
The article itself states that the oxidizing power of perbromates is slugish but more pronounced in power then perchlorates, upon long term storage
the acetone decomposition product was not observed with perchlorates. The authors also reported no unexpected explosions although they prepared for
them, showing somewhat unexpected stability.
|
|
|
Nerro
National Hazard
   
Posts: 596
Registered: 29-9-2004
Location: Netherlands
Member Is Offline
Mood: Whatever...
|
|
I always thought it was logical that BrO4- would be more stable than ClO4- because the Br is som much larger it can spread the electrons more equally
over its orbitals (larger orbitals).
Could a synthesis using Ba(BrO4)2 and Ba(EtSO4)2 be be used to create Et-O-BrO3?
Or perhaps we could modify such a synth to create CH3-(CH2)2-O-BrO3 or even CH3-(CH2)3-O-BrO3. maybe (CH3)2=CH-O-BrO3 would be more stable.
Interesting.
We should find a way to stabilize the product or at least slow down the rate of decomposition significantly because like this it wouldn't be much
use.
#261501 +(11351)- [X]
the \"bishop\" came to our church today
he was a fucken impostor
never once moved diagonally
courtesy of bash
|
|
|
BromicAcid
International Hazard
    
Posts: 3253
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
J. Randell, J.W. Connolly, and A.J. Raymond, J. Amer. Chem. Soc, 83, 3958 (1961).
Stabilization of n-alkyl perchlorates through urea inclusion processes along with preparations for some longer chain derivatives and their properties
including the expected increased stability.
Attachment: ja01480a007.pdf (374kB)
This file has been downloaded 1503 times
|
|
|
Quince
National Hazard
   
Posts: 773
Registered: 31-1-2005
Location: Vancouver, BC
Member Is Offline
Mood: No Mood
|
|
I was wondering if there are any estimates on the performance of ethyl perchlorate (VoD, energy released). Not that I plan to attempt synthesis...
Also, whom do I contact regarding problems with forum software?
\"One of the surest signs of Conrad\'s genius is that women dislike his books.\" --George Orwell
|
|
|
BromicAcid
International Hazard
    
Posts: 3253
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Problems with forum software should be addressed in 'Forum Matters' or U2U'ed to an admin.
As for the energy released via detonation of ethyl perchlorate, I have a PDF entitled "The relationship between performance and constitution of
pure organic explosive compounds." Which lists ethyl perchlorate as having a power and brisance 120% of TNT. The curves shown in the PDF are
maxed out with entries such as 2,4,6-Trinitro-1,3,5-triazidobenzene, Trimethylolnitromethane trinitrate, Azidoethyl nitrate, and
Cyclotrimethylenetrinitramine.
Edit: Link
[Edited on 2/18/2005 by BromicAcid]
|
|
|
Quince
National Hazard
   
Posts: 773
Registered: 31-1-2005
Location: Vancouver, BC
Member Is Offline
Mood: No Mood
|
|
Thanks. Any chance you can email me the PDF?
[Edited on 17-2-2005 by Quince]
\"One of the surest signs of Conrad\'s genius is that women dislike his books.\" --George Orwell
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
I had the oportunity to see some barium ethyl sulfate today, 1lb, electronic grade, however it is very old and very hydrated, the bottle says .2H2O,
but it is a putty. It also has a very unusual smell. Could overtime it somehow turn to diethyl sulfate? I know the smell is familiar, but no idea
from where. It is the same smell that I encountered in the liquid in which cerium metal was stored under.
Its mine if I want it, but not if it is contaminated with diethylsulfate or something nasty.
EDIT: It kinda reminds me of the smell of ethyl borate.
[Edited on 26-5-2006 by rogue chemist]
|
|
|
Swany
Hazard to Others
  
Posts: 188
Registered: 11-4-2005
Location: My happy place...
Member Is Offline
Mood: Sanguine
|
|
I added roughly 10mls of freshly distilled presumed to be mostly pure EtOH to roughly 20mls of H2SO4, and began titration with BaCO3, first whiff I
got when I added some BaCO3 nearly knocked me off my feet! It was utterly foul! Not like rotting flesh, but an industrial bitter sulfur
containing foul- the smell of ethyl sulfate?
Either way, I will attempt it today and I am half-expecting an explosion due to contaminants in my stuff. It shall be as scaled down as I can get it.
EDIT, and now I added some water as it was not neutralizing well, and lo and behold, it boiled over.... welll, I guess I needed some BaSO4 anyways.
Divine intervention, perhaps? 
[Edited on 1-7-2006 by Swany]
|
|
|
dunmail
Harmless

Posts: 6
Registered: 16-7-2007
Location: UK
Member Is Offline
Mood: damp
|
|
I note from this MSDS https://fscimage.fishersci.com/msds/13460.htm that it's possible to make ethyl perc. from Mg(ClO4)2 + EtOH - does anyone know if this is true
please?? Not that I want to make any (I value my body parts too much), it just struck me that it could be an accident waiting to happen if you're
trying to dry anything moistened with an EtOH/water mix over the Mg perc., as I may be wanting to do soon.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Marvin
@ - JohnWW,
Organic perchlorate compounds, with the exception of salts are far too unstable to have any use as explosives. |
The perchlorate radical is more stable than even the nitro group , and more
resistent to degradation from ionizing radiation , to the extent that certain
perchlorate explosives have found use in nuclear weapon implosion assemblies.
( Don't ask for references on this just take it on faith )
Aromatic triperchlorates of benzene ring analogs such as TNB comes to mind.
.
|
|
|
BromicAcid
International Hazard
    
Posts: 3253
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Dunmail, magnesium perchlorate was at one time used frequently to dry gases. It has a very high affinity for water so it's really good for that.
Problem is that it can pick up acid from exit gases and can release the free acid. Couple the free acid with an alcohol and dehydrating conditions
and something like ethyl perchlorate can form, recovery though would be another issue. Suffice it to say, magnesium perchlorate shouldn't come into
contact with organics.
And also, Tito-o-mac, there is a separate thread for the discussion of perchlorates/perchloric acid in general that would be better suited to your
question.
|
|
|
tito-o-mac
Hazard to Others
  
Posts: 117
Registered: 30-6-2007
Member Is Offline
Mood: No Mood
|
|
Blaster, do you have a degree in chemistry/pyrotechnics or both?
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
I thought about where to stick this ( don't be rude ) listed here
are other threads on perchlorate compounds I find interesting.
- Urea Perchlorate
http://www.sciencemadness.org/talk/viewthread.php?tid=3286&a...
- Hexamine Diperchlorate
http://www.sciencemadness.org/talk/viewthread.php?tid=364&am...
- Hexamethylenetetramine Dinitrate ( cites also the perchlorate )
http://www.sciencemadness.org/talk/viewthread.php?tid=885&am...
- Perchlorate compositions
http://www.sciencemadness.org/talk/viewthread.php?tid=6334&a...
Rosco Bodine made the observation that Trimethylolmelamine can be a good
candidate for accepting nitrate groups. I posted some data immediately following
this item here _
http://www.sciencemadness.org/talk/viewthread.php?tid=173#pi...
Immediately after is a post by Axt on how to obtain this other _
[img]http://pubchem.ncbi.nlm.nih.gov/image/imgsrv.fcgi?t=l&cid=62361[/img]
" Hexamethylolmelamine may be produced either by heating melamine with an
excess of neutral formaldehyde to 90" C. (194" F.), or by allowing the melamine
to react with neutral formaldehyde at room temperature over a period of 15 to
18 hours. Elemental analysis indicates that the product formed in both cases is
the same and contains one molecule of water of crystallization per molecule of
hexamethylolmelamine. good yields when melamine is reacted with neutral or
slightly alkaline formaldehyde, or with some substance producing formaldehyde."
I see no reason to be limited only to a nitrated variant and the perchloro group
achieves near ideal oxygen balance. Detonation products amount to 24 mols of
gas per mol. Its physical properties are as yet a matter for speculation.
- Click image for enlargement -
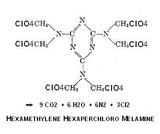
Thoughtful precaution is needed in the use of perchloric acid , the anhydride
unlike nitric acid , is a very reactive high explosive all by itself. Read about safety
concerns here _
http://www.fq.uh.cu/dpto/qi/q_inor_2_web/halogenos/HClO4.htm
http://www-safety.deas.harvard.edu/advise/PerchloricAcid.htm...
Other thread of this forum here _
http://www.sciencemadness.org/talk/viewthread.php?tid=12&...
Using an inorganic salt of the mineral acid buffers synthesis by ion metathesis ,
obviating the shortcomings from the direct action of the acid. This can serve
to confidently prepare a variety of otherwise difficult to make compounds.
Saturated aqueous solutions of Hydrazine Sulphate ( ~ 200 gm/ 100 ml water )
and Magnesium Perchlorate ( ~ 100 gm/ 100 ml water ) mixed together cold
should precipitate Magnesium Sulphate decahydrate leaving a concentrated
solution of Hydrazine Perchlorate _
Aqueous (H2NNH2)2:H2SO4 + Mg(ClO4)2 -> MgSO4.10H2O + 2 H2NNH2.HClO4
What I have just outlined here goes by another name when it is concentrated ,
it is also known as Astrolite. While there are of course materials which are much
less safe to work with , a healthy respect for it will ensure your continued ability
to experiment without maiming disabilities. Be aware also that a general rule of
shock sensitive liquid explosives is that the presence of minute bubbles makes it
much more sensitive to much less of a shock. In this condition anhydrous Astrolite
liquid is reportedly " as sensitive as ethylene glycol dinitrate " that's a direct quote.
I'm reminded of another quote I read , written by Gerald Hurst , Cheif scientist
at the Atlas Powder company that developed Astrolite ,
" Several fellows discovered new ways to detonate it , but they're not around
to tell how they did it. Astrolite will blow up for it's own reasons and it is the
reasons you don't know that will kill you. Never try to make Astrolite of any kind
in glasssware unless , you're tired of living. "
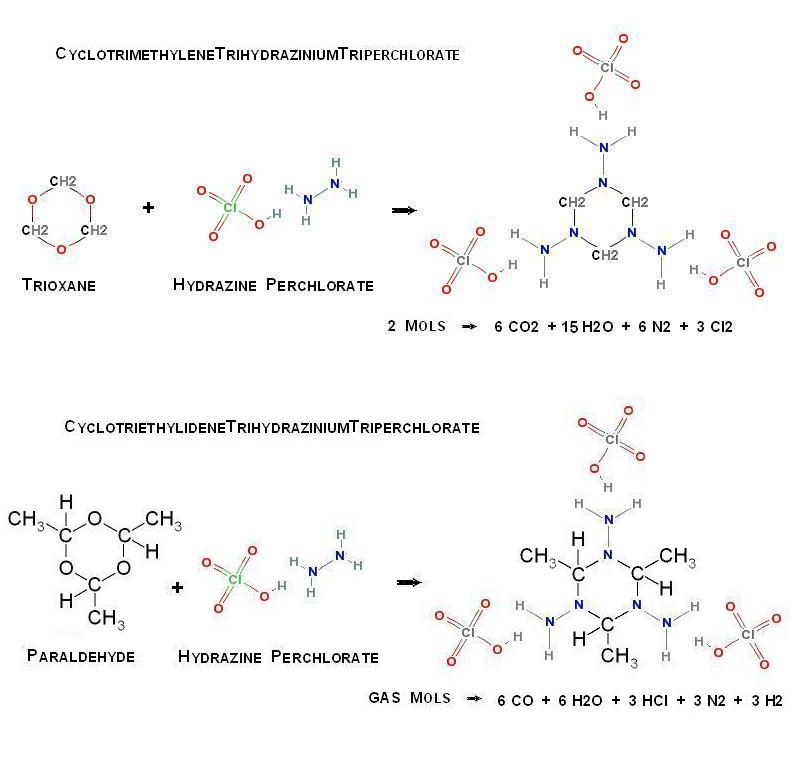
Possible new organic perchlorate salt
So now that you have Hydrazine Perchlorate , what to do with it ?
Analogously to the formation of HexamethyleneTetramine , I wonder if by the
introduction of Trioxane this can be further condensed in the following way _
(CH2O)3 + H2NNH2.HClO4 -> (CH2)3(NNH2.HClO4)3
Alternatively , Paraldehyde may similarly be substituted
The supposed structures are pictured here below _
- Click image for enlargement -

ArgusLab file here _ http://www.badongo.com/file/4666745
[Edited on 10-10-2007 by franklyn]
|
|
|
| Pages:
1
2
3
4
5
..
7 |