| Pages:
1
2 |
teodor
National Hazard
   
Posts: 924
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
ethanol self-condensation
There is a popular method of ethanol purification by reflux with alkali (KOH/NaOH) and distillation which cause self-condensation of ketones etc.
Usually after this process the residue has a deep brown color of condensation products. But I found that after keeping this residue (10% in KOH by
mass) several monthes and distilling-off ethanol (in vacuum) new addition of KOH/keeping overnight at room temperature makes the vacuum-distilled
ethanol yellow/brown again.
So, it looks like there is some self-condensation of EtOH but I didn't find any information about that.
What is the reaction?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4356
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
That orange stuff also happens in ethanol base-baths.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
I believe that the first step is something to the effect of:
EtO- + O2 >> EtO3- >> HO2- + AcH
You can probably guess what might come next.
|
|
|
SplendidAcylation
Hazard to Others
  
Posts: 203
Registered: 28-10-2018
Location: Starving in some deep mystery
Member Is Offline
Mood: No one I think is in my tree.
|
|
HO2- + AcH -> AcOH + OH-
AcOH + OH- -> AcO- + H2O
Perhaps something like this? Also is HO2- a stable species? I couldn't find much about the conjugate base of hydrogen peroxide, just the HO2. radical.
|
|
|
teodor
National Hazard
   
Posts: 924
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
Clearly_not_atara, this hypothesis is quite logical but lacks an experimental evidence: when I put Na2SO3 together with KOH it doesn't inhibit the
reaction but even I observe the color change to yellow faster. Also, my base bath looks like the reaction stops after some saturation: the liquid
doesn't turn darker with time.
But also my experiment with sulfite and assumptions could be wrong.
The process goes only at high alkali concentration, most readily when you have undissolved KOH. If I dissolve the same amount of alkali the change of
color doesn't occure. It looks like the reaction proceeds more rapidly near the surface of KOH.
Update. Oh, I see what could be wrong in my experiment. Sodium sulfite doesn't dissolve in ethanol. Have to find another reducer.
[Edited on 18-7-2023 by teodor]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Scavenging oxygen with sulfite is likely to be difficult because sulfite does not react that much more quickly (if at all) than ethoxide. You might be
able to generate an inert atmosphere by scrubbing with bisulfite before forming the ethoxide, but it sounds like a complicated setup. Easier would be
to use a nitrogen atmosphere.
Ethoxide is most commonly made using Na metal which acts as a very strong oxygen scavenger and generates a briefly stable solution.
More efficient from a practical perspective is to rapidly precipitate the ethoxide by adding acetone so that it can be stored as a solid and used when
needed.
| Quote: | HO2- + AcH -> AcOH + OH-
AcOH + OH- -> AcO- + H2O
Perhaps something like this? Also is HO2- a stable species? |
HO2- is more likely to react with the ethanol present than to attack the very small amount of acetaldehyde that exists at equilibrium. Acetaldehyde
likewise will interact with ethoxide by aldol condensation rather than immediately undergoing a BV.
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Uh, no... Not your call, and this is a worthwhile discussion. The paper you linked is interesting in its
own right, but not really relevant since we are talking about the interaction of sodium ethoxide with ethanol and its decomposition in solution, while
the paper discusses decomposition of solid sodium alkoxides in air. These are not equivalent processes. Being in solution in ethanol clearly leads to
some weird things that can't be explained by simple hydrolysis or absorption of CO2. Note that even the commercial NaOEt in the picture in
the paper you linked is yellowish, while the NaOMe is pure white.
Air-oxidation of ethoxide to acetaldehyde followed by aldol condensation with itself, as atara suggests, sounds quite plausible. This is an option
that isn't available for methoxide, which would explain the lack of discoloration seen for NaOMe.
|
|
|
kmno4
International Hazard
    
Posts: 1503
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
| Quote: | | (...) we are talking about the interaction of sodium ethoxide with ethanol and its decomposition in solution, while the paper discusses (...)
|
No.
You are talking abot something getting brown. EtOH p.a. and better does not change its coloration with KOH/NaOH. There is nothing to discuss about.
Слава Україні !
Героям слава !
|
|
|
DraconicAcid
International Hazard
    
Posts: 4356
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by kmno4  | | Quote: | | (...) we are talking about the interaction of sodium ethoxide with ethanol and its decomposition in solution, while the paper discusses (...)
|
No.
You are talking abot something getting brown. EtOH p.a. and better does not change its coloration with KOH/NaOH. There is nothing to discuss about.
|
Have you ever worked with basic ethanol? It does indeed turn orange/brown on long exposure to air.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
teodor
National Hazard
   
Posts: 924
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
I see, EtO- is an ionized form. So, the oxidation speed should be dependent on ion concentration. I am thinking how to demonstrate that.
The point of my post is that there is a common belief that the yellow color in ethanol + alkali is because of ethanol impurities, but I think it is
not impurities, the process goes with pure ethanol as well.
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by kmno4  | | You are talking abot something getting brown. EtOH p.a. and better does not change its coloration with KOH/NaOH. There is nothing to discuss about.
|
Is that so? How much do you want to bet on that? I’ll test that claim today.
|
|
|
Bedlasky
International Hazard
    
Posts: 1243
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
I have some ketone free EtOH (denaturated with heptane, Et3N and denatonium benzoate, so this shouldn't interfer). So I can do some experiments this
weekend - I'll prepare NaOH solution in EtOH, one part of solution will be stored under nitrogen, second part under air and lets see what will
happen.
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
I already have some ACS grade KOH sitting in USP grade absolute ethanol on my bench. We’ll see how it’s looking tomorrow.

|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Quote: Originally posted by kmno4  | No.
You are talking abot something getting brown. EtOH p.a. and better does not change its coloration with KOH/NaOH. There is nothing to discuss about.
|
The paper you referenced contains a picture of sodium ethoxide in a jar. It is tan. Did you read it?
Attached, for reference.
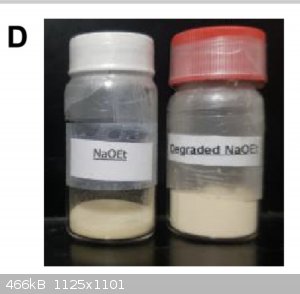
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Well, would you look at that, 24 hours later, it’s yellow.

Care to comment, kmno4?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4356
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I'm actually surprised the colour change was noticeable after only one day. Leave it exposed to air, and we'll see how long it takes to turn orange.
[Edited on 20-7-2023 by DraconicAcid]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
AvBaeyer
National Hazard
   
Posts: 651
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Search the Literature!
Perhaps the attached paper will provide the insight to reach an answer. It is amazing what one can find by search a bit - like 10 minutes.
AvB
Attachment: STABILIZATION OF THE ALCOHOLIC POTASSIUM HYDROXIDE.pdf (397kB)
This file has been downloaded 240 times
[Edited on 21-7-2023 by AvBaeyer]
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Thanks, AvB. Yes, searching the literature is generally a great idea. Nothing wrong with running an easy experiment (which took me far less than 10
minutes to set up) to refute the naysayers, though. The paper you linked supports the oxidative degradation hypothesis, but doesn't give any further
insight on what is actually happening to the ethanol or why this degradation occurs for ethanol but seemingly not for isopropanol. I tried searching
too, but was unable to find any direct studies of the phenomenon, and I don't have time to do a more thorough search.
|
|
|
teodor
National Hazard
   
Posts: 924
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
Thanks for the paper, AvBaeyer.
They mention they tried hydroquinone. It's very strange, because mixing Hydroquinone, KOH and EtOH produce a yellow color _immediately_.
|
|
|
Bedlasky
International Hazard
    
Posts: 1243
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
I decided to check if EtOH is truly oxidized by O2 in alkaline environment to acetaldehyde. I used Schiff's reagent to test it. I have some leftover
solution, which is several months old, so I firstly tested if it is ok using 5% formaldehyde. Reagent produced strong red coloration, so it's still
usable.
I dissolved 2 g of NaOH in 75 ml of 95% ketone-free EtOH. I bubbled air through it for 2h. Then I added 50 ml of water and 1 ml of sample (H2O, EtOH,
NaOH+EtOH) in to 3 beakers and addjusted pH to 3 using dilute H2SO4. After that I added in to each beaker 1 ml of Schiff's reagent. After 10 minutes
beaker with NaOH+EtOH sample was slightly pink in colour. Colour was weak but still clearly visible. Beakers with EtOH and H2O samples were
colourless.

|
|
|
teodor
National Hazard
   
Posts: 924
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
Thanks Bedlasky. Your experiment clearly proves clearly_not_atara hypothesis.
Meanwile I checked sodium dithionite, Na2S2O4. It is slightly soluble in ethanol and really can delay degradation. But it doesn't prevent it
completely.
Also reaction of hydroquinone and alkali gives very similar results both by color development and by the mechanism (air oxidation), but proceed
much-much faster, so I probably will use it as a test for the power of oxygen scavengers. Na2S2O4 also delays the reaction of air oxidation of alkali
hydroquinone, but doesn't stop it completely.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Thanks Bedlasky!
|
|
|
Bedlasky
International Hazard
    
Posts: 1243
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
I repeated experiment today, this time using more soluble KOH and 4 h reaction time. I dissolved sample only in 25 ml of water, addjusted to pH 3 with
sulfuric acid and added Schiff's reagent. After few minutes nice pink colour developed in the solution.
That water blank looks yellow on the photo, but it's actually colourless in reality.

[Edited on 23-7-2023 by Bedlasky]
|
|
|
Rainwater
National Hazard
   
Posts: 937
Registered: 22-12-2021
Member Is Offline
Mood: Break'n glass & kick'n a's
|
|
This might be relevant
Translated version
https://www.scielo.br/j/jbchs/a/SnNtvb6zqPZpSTYr3T5STTK/?lan...
Orginal https://doi.org/10.1590/S0103-50531997000500002
Atmospheric chemistry of alcohols
"You can't do that" - challenge accepted
|
|
|
Rainwater
National Hazard
   
Posts: 937
Registered: 22-12-2021
Member Is Offline
Mood: Break'n glass & kick'n a's
|
|
Back in February I placed some MgSO4 and freshly distilled 96% ethanol into test tubes to dry.
I dont need much each a time so I usually make a bunch of tubes (20ml), use what i need and discard the rest.
Monday, my last 2 tubes where crystal clear, today they are tented yellow.

MgSO4 was obtained at the pharmacy as a oral laxative, dried in a vacuum at 400c for 2 hours, it should be pure.
"You can't do that" - challenge accepted
|
|
|
| Pages:
1
2 |