| Pages:
1
..
30
31
32
33 |
Simoski
Hazard to Self
 
Posts: 82
Registered: 24-12-2017
Location: Johannesburg South Africa
Member Is Offline
Mood: No Mood
|
|
It's like Russian to me too. 
But they seem to be making progress
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
On the preceding page I posted this interesting compound
and have attached the related Journal article which describes the compound at the bottom of page 321 of the article.
Quote: Originally posted by Rosco Bodine  |
Here is an interesting compound where hexamethylene tetramine is shown to bridge complex 2 copper compounds, and such a scheme might work for copper
DDNR similarly as it does for copper azide and may be useful as a desensitizer integrated on a molecular level. Other metal salts might also be
susceptible.
For the complex if it would form with copper DDNR, there would be 4 of the mono-acid DDNR molecules in a compound having 2 copper atoms bridged by
hexamethylene tetramine. If the hypothetical compound forms, it may be possible to use the benign ammonium DDNR as a precursor. Whether the speculated
compound will form at all or whether any troublesome hydrate may be an issue is completely unknown, since the hypothetical compound is unreported and
may be novel.
Glycine should similarly bridge complex 2 copper molecules, and may work better for the same purpose.
|

Attachment: php0embtA (1.1MB)
This file has been downloaded 829 times Journal_für_praktische_Chemie 1943 pg 307-328 hexamine complexed copper azide
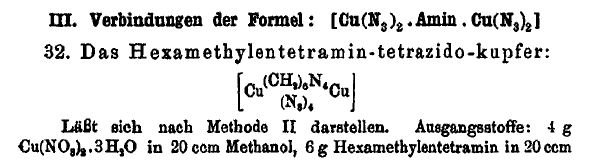 
The solubility in ammonia makes this compound interesting for its potential to form a double salt or mixed salt with glycine or hexamine complexed
copper perchlorate. Even a cocrystallized mixture could have interesting enhanced properties for incorporation of the azide content.
[Edited on 10/13/2019 by Rosco Bodine]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Dipicric acid or DIPAM can be obtained in low yield by direct nitration of 3'3-dihydroxybiphenyl or in very good yield by oxidation of
3,3'-Dimethyl-2,2',4,4',6,6'-Hexanitrobiphenyl. Reference: "ADOLPH, HORST G., JOSEPH C. DACONS, and MORTIMER J. KAMLET. Heat Resistant Explosives. XI.
An Unusual Oxidation Reaction Leading to 3, 3'-Dihydroxy-2, 2', 4, 4', 6, 6'-Hexanitrobiphenyl (Dipicric Acid, DIPA). No. NOLTR-62-32. NAVAL ORDNANCE
LAB WHITE OAK MD, 1962."
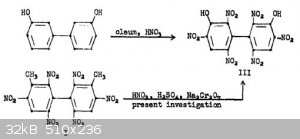
Would it be possible to selectively reduce a single nitro group on one of the rings using the copper/ascorbic acid reduction scheme due to immediate
precipitation as the cuprous salt? Would the resulting mono or diamine derivative be able to diazotize forming a DDNP molecule bridged to a picric
acid ring or two DDNP molecules attached to each other? Would the former be able to form salts? Or would the biphenyl bond in such a molecule be very
unstable/susceptible to hydrolysis?
[Edited on 20-9-2020 by nitro-genes]
|
|
|
garphield
Hazard to Self
 
Posts: 58
Registered: 9-12-2019
Member Is Offline
|
|
Picramic acid can act as a base, i.e. with isopicramic sulfate forming when picramic acid is combined with sulfuric acid. Could isopicramic
perchlorate be a viable energetic? The perchlorate anion would make the OB a bit better, although it still would be far from 0.
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 443
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
DDNP/Diazo
This thread sort of expanded from DDNP into theoretical diazo heterocycles in search of unknown primary explosives. I've read through the thread and I
dont think there was an Ullmanns reference but I'm not going to read through it again to double verify it. So here is Diazo industrial preparation pdf
that explains a bit about practical behavior and character of diazo materials with a perspective of dye stuff production.
Attachment: Diazo Compounds Ullmanns.pdf (621kB)
This file has been downloaded 367 times
There is also recent interest and discussion of complexes in the thread so in support of readers interested in that topic, these are about inorganic
diazo and dinitrogen complexes and organic diazo heteroaromatics.
Attachment: diazo metal ligand complexes.pdf (2.8MB)
This file has been downloaded 363 times
Attachment: prep of diazo compounds.pdf (2.7MB)
This file has been downloaded 326 times
Attachment: Dinitrogen Transition Metal Complexes.pdf (1.1MB)
This file has been downloaded 362 times
[Edited on 26-12-2022 by Hey Buddy]
|
|
|
Raid
Hazard to Everyone
  
Posts: 203
Registered: 14-11-2022
Location: N/A
Member Is Offline
|
|
Is there any good papers for the deacetylation of picric acid?
And DDNP synnthesis?
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
There is a 10 page thread dedicated to what you have just asked http://www.sciencemadness.org/talk/viewthread.php?tid=433
I suggest you also read this thread that you have just posted in, it contains all of the information that you need.
|
|
|
Raid
Hazard to Everyone
  
Posts: 203
Registered: 14-11-2022
Location: N/A
Member Is Offline
|
|
Thanks for the info 
|
|
|
Raid
Hazard to Everyone
  
Posts: 203
Registered: 14-11-2022
Location: N/A
Member Is Offline
|
|
I am not sure if this info has been posted on another thread, but If you're looking for potassium or sodium nitrite for your DDNP synthesis then I
have a solution for you!
It's very simple. Get some potassium or sodium nitrate and heat it in a metal cup with a blowtorch under direct flame until molten and no more oxygen
bubbles appear.
I have not gotten the exact temp at which the nitrate salt decomposes to form nitrite but it is said to be from 550c to 790c. A blowtorch should be
able to get to that temp with no problem. Also if you're wondering what gas I was using it was just propane without any added oxygen.
Some more things to consider when making the sodium nitrite. make sure you use a METAL reaction vessel aluminum will not work because it would react
with the molten KNO3 or NaNO3 and decompose further than it should have. Also make sure to clean your reaction vessel very very very well. If there is
even a small amount of contaminants it will show up in your final product and will be very hard to get out.
Personally I think this method is much better then all of the other methods on the thread about nitrate reduction and is MUCH faster and will save you
loads of time and reagent because the yield is nearly 100%, probably around 97% or something.
Note to Admins and Mods:
I'm guessing someone will try to move this post over to the thread about nitrate reduction, but I would appreciate it if you kept it here or made a
copy and put it on that thread. I'm saying this because I think this is valuable information to the people that are wanting to make DDNP and are
having a hard time getting or making a nitrite salt. Also the thread about nitrate reduction is old and hard to find. This info would be much more
convenient for people to look on here and find what they are looking for.
|
|
|
ManyInterests
National Hazard
   
Posts: 966
Registered: 19-5-2019
Member Is Offline
|
|
Quote: Originally posted by Raid  | I am not sure if this info has been posted on another thread, but If you're looking for potassium or sodium nitrite for your DDNP synthesis then I
have a solution for you!
It's very simple. Get some potassium or sodium nitrate and heat it in a metal cup with a blowtorch under direct flame until molten and no more oxygen
bubbles appear.
I have not gotten the exact temp at which the nitrate salt decomposes to form nitrite but it is said to be from 550c to 790c. A blowtorch should be
able to get to that temp with no problem. Also if you're wondering what gas I was using it was just propane without any added oxygen.
Some more things to consider when making the sodium nitrite. make sure you use a METAL reaction vessel aluminum will not work because it would react
with the molten KNO3 or NaNO3 and decompose further than it should have. Also make sure to clean your reaction vessel very very very well. If there is
even a small amount of contaminants it will show up in your final product and will be very hard to get out.
Personally I think this method is much better then all of the other methods on the thread about nitrate reduction and is MUCH faster and will save you
loads of time and reagent because the yield is nearly 100%, probably around 97% or something.
Note to Admins and Mods:
I'm guessing someone will try to move this post over to the thread about nitrate reduction, but I would appreciate it if you kept it here or made a
copy and put it on that thread. I'm saying this because I think this is valuable information to the people that are wanting to make DDNP and are
having a hard time getting or making a nitrite salt. Also the thread about nitrate reduction is old and hard to find. This info would be much more
convenient for people to look on here and find what they are looking for. |
Making nitrite has been a dirty and messy process I find. Thankfully I found a seller from Poland that sells sodium nitrite. I bought 200 grams from
them and that should be enough for me for a good long time.
Edit: just some questions about para and ortho-DDNP. Can they be loaded into stainless steel bodies? Also when loaded. How sensitive are they to being
pressed by a vise? I did hear from a user that a few drops of methanol is needed to desensitize them before pressing and allowing them to cure in the
cap before inserting a fuse/electric match. Is this correct?
[Edited on 27-5-2023 by ManyInterests]
|
|
|
Ankit1612
Harmless

Posts: 32
Registered: 2-11-2022
Member Is Offline
|
|
Hi guys,
I want to synthesis DDNP and i don't have Potassium or Sodium nitrite.
So, i want to know that if i can use nitrate salt or Nitric acid?
|
|
|
Raid
Hazard to Everyone
  
Posts: 203
Registered: 14-11-2022
Location: N/A
Member Is Offline
|
|
you can use RFNA or WFNA
|
|
|
Raid
Hazard to Everyone
  
Posts: 203
Registered: 14-11-2022
Location: N/A
Member Is Offline
|
|
Quote: Originally posted by ManyInterests  |
Edit: just some questions about para and ortho-DDNP. Can they be loaded into stainless steel bodies? Also when loaded. How sensitive are they to being
pressed by a vise? I did hear from a user that a few drops of methanol is needed to desensitize them before pressing and allowing them to cure in the
cap before inserting a fuse/electric match. Is this correct?
|
I would think that it would be safer to use MeOH or EtOH to desensitize the the DDNP but I have never needed to do this before, The DDNP seems to be
relatively non-sensitive to Newtonian forces. And yes, DDNP can be packed into stainless steel without compatibly issues. Furthermore DDNP is
compatible with almost all transition metals and the DDNP will remain non-effected in contact with these metals for long periods of time.
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 494
Registered: 12-10-2022
Location: [REDACTED]
Member Is Offline
Mood: Still in 2022
|
|
Umm...I'm pretty sure a diazotization requires a nitrite.
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Really? How do you perform a diazotization with nitric acid?
Edit: sorry Sir Gawain I did not see your post
[Edited on 18-6-2023 by B(a)P]
|
|
|
Microtek
National Hazard
   
Posts: 920
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Yes, you can use nitric acid or sulfuric acid with a nitrate salt for the nitration of a phenol in the production of picric acid. On a related note,
you can't deacetylate picric acid, since there is no acetyl group to remove. I suppose the comment was about deacetylizing acetylsalicylic acid.
|
|
|
Ankit1612
Harmless

Posts: 32
Registered: 2-11-2022
Member Is Offline
|
|
Actually i already have Picric acid which i made by nitration of phenol.
And i want of make DDNP out of it! But i don't have nitrite salt and making nitrite from nitrate is very messy work!!!
That's why i'm seeking for any other method which does'nt need nitrite salt.
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 494
Registered: 12-10-2022
Location: [REDACTED]
Member Is Offline
Mood: Still in 2022
|
|
You have to use a nitrite.
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
Microtek
National Hazard
   
Posts: 920
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
I used a method to produce (organic) nitrite by reducing dilute (ca. 50%) nitric acid with potato starch under heating. This produces a mix of
nitrogen oxides that is equivalent to N2O3 which, when absorbed in water produces 2 molar equivalents of HNO2. I absorbed the gas in aqueous
isopropanol with ice cubes to produce isopropyl nitrite, but you could lead it into NaOH to produce sodium nitrite. You can also use metallic copper,
and doubtless many other things as well, to reduce the nitric acid, but you need the correct concentration of nitric.
|
|
|
Ankit1612
Harmless

Posts: 32
Registered: 2-11-2022
Member Is Offline
|
|
This method is looking very difficult!
Any other easy method to make nitrite?
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 494
Registered: 12-10-2022
Location: [REDACTED]
Member Is Offline
Mood: Still in 2022
|
|
This was recently posted on the 'Preparation of Ionic Nitrites' thread:https://www.youtube.com/watch?v=5BLPoE6Y-ns. This is probably the easiest way.
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
Microtek
National Hazard
   
Posts: 920
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Easy, but the product is of unknown purity. The test that he does at the end of that video is indeed qualitative, and does not give any indication of
purity of the product. That is the advantage of using N2O3 to generate an organic nitrite from an aqueous solution of an alcohol - the organic layer
that separates will be practically pure, since the likely contaminant (the nitrate) won't form with this much water in the mix.
You could then react a slight excess of isopropylnitrite with aqueous NaOH to produce pure NaNO2. The water and remaining IPNitrite could then be
evaporated.
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 494
Registered: 12-10-2022
Location: [REDACTED]
Member Is Offline
Mood: Still in 2022
|
|
Yes, but the impure product from almost any method can be purified by conversion to isopropyl nitrite. While your method is simpler and probably far
superior, it uses nitric acid, a chemical very difficult to obtain for beginners.
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
fx-991ex
Hazard to Others
  
Posts: 109
Registered: 20-5-2023
Member Is Offline
|
|
Quote: Originally posted by Raid  |
Some more things to consider when making the sodium nitrite. make sure you use a METAL reaction vessel aluminum will not work because it would react
with the molten KNO3 or NaNO3 and decompose further than it should have. |
What about a glazed porcelain crucible? is it ok?
[Edited on 22-6-2023 by fx-991ex]
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Quote: Originally posted by fx-991ex  | Quote: Originally posted by Raid  |
Some more things to consider when making the sodium nitrite. make sure you use a METAL reaction vessel aluminum will not work because it would react
with the molten KNO3 or NaNO3 and decompose further than it should have. |
What about a glazed porcelain crucible? is it ok?
[Edited on 22-6-2023 by fx-991ex] |
Glazed porcelain would be fine. I would avoid a metal reaction vessel.
|
|
|
| Pages:
1
..
30
31
32
33 |