| Pages:
1
..
6
7
8 |
Osmiridium
Harmless

Posts: 26
Registered: 13-3-2023
Location: Multiverse
Member Is Offline
Mood: excited
|
|
Very fascinating and beautiful these violuric acid salts! Something I will certainly do aswell.
So cool your thiovioluric acid salts, Ormarion!
I would be very interested in the selenium and tellurium derivates.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Welcome to the board, Osmiridium.
I am curious at this point -- what is it about this particular anion that gives rise to such a range of different colours. This is unusual behaviour.
|
|
|
Cakethegrate
Harmless

Posts: 4
Registered: 8-12-2022
Member Is Offline
|
|
Quote: Originally posted by j_sum1  |
I am curious at this point -- what is it about this particular anion that gives rise to such a range of different colours. This is unusual behaviour.
|
Hey j_sum, that is a very good question that I would love to attempt to explain/answer, apologies for the slightly late response.
Color may seem very simple in concept, however when you take into consideration as to what is actually happening at a molecular level and what causes
these colors it tends to become very tricky...
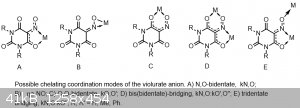
Violuric acid and its derivatives are very interesting and peculiar in many ways, for example, it very often forms supramolecular compounds,
especially its organoammonium salts, which entail vast networks of complex hydrogen bonded networks. It is also a chelating acid which can be either
monodentate, bidentate, or tridentate(depending on the counterion that it is reacted with) and has many different chelating modes which I will show
with the structures below
The colors of the exact same violurate salt will often even be different colors or shades completely depending on reaction conditions, ie how it is
precipitated, the pH of the solution when it is precipitated, what solvent the reaction is carried out in etc.
All of these factors can take a big part in the colors of violurates, however, it is even more important to take into consideration what is happening
when you are forming a violurate salt. It is clear that it is the salt formation that is at least partially responsible for its color, as the violuric
acid itself is colorless. The factor that I believe to be most responsible for the vast and vibrant colors of the violurates has to do with violuric
acid's tautomerism and intermolecular interactions.
If you have ever made violurates before you have likely noticed a very odd interaction happens when the colorless acid is dissolved in water(or any
protic solvent for that matter), it immediately turns the solution a very pleasing pastel pink color. I have heard many people think that this sudden
color change is due to some sort of contamination being introduced, as the violuric acid is seemingly reacting with nothing, however that is not true.
This light pink color is in actuality caused by the proton from the oxime(NOH) tautomerizing to one of the surrounding two ketones creating a nitroso
group(N=O), and a COH group, as seen below.
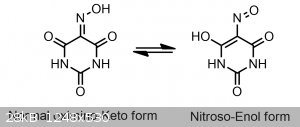
This more acidic nitroso-enol tautomer is indeed also responsible for the color that we observe with violurates. If you were to make a violurate salt
that was only composed of a salt made from the normal oximino-keto form, it would likely be colorless, yellow or a dim orange.
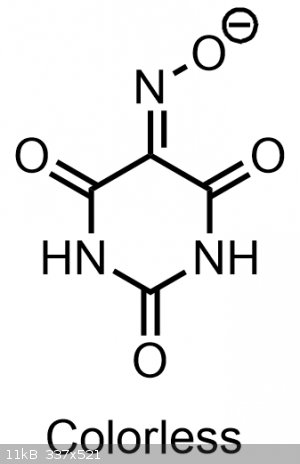 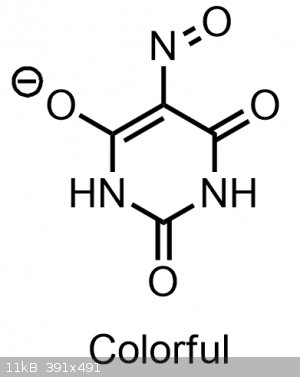
Normally this more colorful, acidic, and strained nitroso-enol form is not favored(which is why it doesn't exist/isn't observed in the solid state in
violuric acid and it is definitely the less occurrent tautomer when dissolved in water, though enough is still formed in solution to produce an
observable color change) When a base is added to the solution on the other hand, this pushes the equillibrium towards the more strained, yet more
acidic nitroso-enol form. Naturally, because of the nitroso-enol form's strain, it completely alters the absorbance of the salt that is formed, which
equates to the highly varied and vibrant colors that we all know and love.
Anyways, if you have any questions or confusion that you would like to have cleared up, I would love to answer them.
-Cake <3
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Those last two structures you've drawn aren't tautomers, but resonance structures, and you can't convince me that they'll have different colours.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Let me paraphrase and see if I have this right.
The colour of volurate salts come from the conjugated double bonds when it resonates into an enolic form. The precise hue depends on both the geometry
and the strength of the cation. The geometry is further affected by the possibility of differing chelation states and may also be affected by
variations in crystalline structure arising from the conditions under which it crystallised.
I am not sure whether to agree with DA that we are talking about resonance not tautomerism here. Tautomerism involves movement of protons with the
solvent as an intermediary in my understanding.
The ketone structure does not jump out at me as one that is likely to be coloured. But that could well be my ignorance.
|
|
|
Cakethegrate
Harmless

Posts: 4
Registered: 8-12-2022
Member Is Offline
|
|
Yeah, my mistake, what I was trying to show with the last two are the different salts that would form resulting from the tautomerization, even though
the bonding is certainly more complex than that. Trust me, the last two would very much be different colors, although nitroso compounds are often very
unstable and carcinogenic, they also have some of the most vibrant and beautiful colors resulting from this instability, again, because of the
strained bonds. Whereas the oxime normally isn't a very colorful group. It is well known that the compounds which have true and stable nitroso
bonds(N=O) are often very colorful and vibrant, and is known to influence compounds absorbance greatly, whereas the oxime one moiety(=N-O) which would
otherwise be formed is typically colorless. So yes, their colors would be very different, because bond strain matters a lot with the colors of
violurates. The crystallographic studies on violurates only proves this and that when reacting violuric acid with a base, it is the nitroso-enol
tautomer that is forming the salt which is what gives violurates their color. I would recommend reading these papers that mention this phenomenon with
violurates.
https://journals.iucr.org/m/issues/2019/02/00/yc5016/index.h...
To quote this paper:
"The material designed in our group and chosen for this study –
tyraminium violurate – is an example of how chromic effects
can be tuned through co-crystallization. Both components,
tyramine (TYR) and violuric acid (VA), are colourless solids
and only when combined form colourful multicomponent
systems. Multicomponent materials containing neutral violuric
acid molecules (ketone–oxime form) either lack colour or
form e.g. light yellow or orange crystals. The more acidic
nitroso–enol form of violuric acid is believed to be formed in
solution prior to a reaction between violuric acid and a base"
https://pubs.rsc.org/en/content/articlelanding/2016/dt/c6dt0...
Edit: Sorry I wrote this post before I saw yours j_sum, and my reply was in response to DA, and yes, all papers I have read claims it is tautomerism,
this is supported based on the fact that the violuric acid changes color(from the shifting of the protons) only in solution, and is colourless in the
solid state. So the solvent is an intermediary and plays a large role in the color of the violurates, and it even matters which solvent you use.
Also, I dont believe the ketone form to be colorful either, I think it is the niitroso-enol form that is the main reason for the violurates color.
However my view could certainly be wrong, but the literature I have found on it seems to support it. Your paraphrasal of my proposal is also very good
lol, thanks for helping with clarity.
Any constructive criticism is appreciated.
[Edited on 19-3-2023 by Cakethegrate]
|
|
|
Osmiridium
Harmless

Posts: 26
Registered: 13-3-2023
Location: Multiverse
Member Is Offline
Mood: excited
|
|
Many thanks Cakethegrate for the good and thorough explanation!
Well, I try to explain my opinion regarding tautomerism. It's actually a very good and important question.
This is clearly tautomerism. Why: Tautomerism is a special form of isomerism. So we have actually two different compounds that may
also have different properties and show different behavior like e.g. colors but with the same empirical formula. But what classifies tautomerism is
that one tautomeric isomer transforms relatively easy into the other but the molecule has to rearrange and is different as such and oxidation states
usually change.
Sometimes the tautomers cannot be separated at all, sometimes one of them is more stable under certain conditions and the "pure substance" consists of
only one of them. Best example is the keto-enol tautomerism where the keto form is usually (not always) more stable.
In this case the hydrogen atom (or the negative charge) changes its location to a different atom. That's what happens with violuric acid.
Resonance structures on the other side try do depict a more complex reality that could not be drawn by one single simple formula in certain cases with
delocalized charges. Or you draw it as a so called hybrid structure. But the most important to note there is: It's always about the same
molecule with identical properties and the oxidation states do not change (we have even kind of partial oxidation states, the concept of oxidation
states is only a model anyway). Most prominent examples are aromatic compounds like benzene or the carbonate anion and so on.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
The two forms of the acid are clearly tautomers.
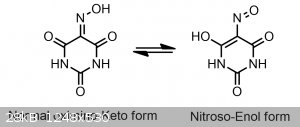
You've moved the hydrogen from one oxygen to another.
Once you take that hydrogen off, then the negative charge gets delocalized, and you have resonance structures. It's not tautomerism- you're not
moving any atoms around. That's just resonance.
I wonder if anyone has done crystal structures of these violurates to see if the bond lengths change with cations and colour....
Well, from the papers cited above, they've obviously done crystal structures...I just wonder if they've come up with an explanation for the different
colours that I can understand.
[Edited on 19-3-2023 by DraconicAcid]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
| Pages:
1
..
6
7
8 |