| Pages:
1
2
3
4 |
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Solubility of copper sulphate compared to its pentahydrate
Despite being an apparently simple topic, the solubility behavior of hydrated salts compared to their anhydrates (and associated anomalies) remain
undocumented and unexplained.
The results of a previous thread I made on this topic were inconclusive and ultimately spiraled into a mess, so I'm narrowing the scope of this post to only look
at copper sulphate and its pentahydrate.
Part 1 - Theory
The solubility in water of any hydrate salt should theoretically be proportional to the total dry mass of salt and the total mass of water, including
the water of solvation and the water contributed by the salt's water of crystallization.
I devised the following formula to compute the solubility of any hydrate (s ₕ) from the solubility of its anhydrate (sₐ) and their molar mass
ratio (Mᵣ):
s ₕ = sₐ/(Mᵣ + sₐ(Mᵣ - 1)/1000)
Where:
s ₕ is the solubility of the hydrated salt in g/L at a given temperature.
sₐ is the solubility of the anhydrous salt in g/L at a given temperature.
Mᵣ is the anhydrate/hydrate molar mass ratio.
The point of this formula is that the mass ratio between the water and salt should remain constant regardless of the salt's hydration state.
| Quote: |
Solved example for copper sulphate:
The molar mass of anhydrous copper sulphate (CuSO₄) is 159.61 g/mol. The molar mass of copper sulphate pentahydrate (CuSO₄·5H₂O) is 249.69
g/mol. Therefore the molar mass ratio (Mᵣ) is 159.61/249.69 = 0.63923.
Let's assume that the solubility of anhydrous copper sulphate (sₐ) is 200 g/L at 0°C. According to the following formula, the solubility of the
pentahydrate (s ₕ) at this temperature should be:
s ₕ = sₐ/(Mᵣ + sₐ(Mᵣ - 1)/1000)
s ₕ = 200/(0.63923 + 200(0.63923 - 1)/1000)
s ₕ = 352.7 g/L
200 grams of anhydrous copper sulphate dissolved in 1 liter, or ~1,000 grams of water is equivalent to a water:salt mass ratio of 1,000/200 =
5.00.
352.7 grams of copper sulphate pentahydrate (CuSO₄·5H₂O) consists of 225.5 grams of copper sulphate (CuSO₄) and 127.2 grams of water (H₂O).
If this mass of copper sulphate pentahydrate is dissolved in 1 liter, or ~1,000 grams of water, the water:salt mass ratio is again equal to
(1,000+127.2)/225.5 = 5.00. |
Part 2 - Empirical data
Copper sulphate was chosen as the subject for this thread because of its relatively simple hydration behavior (as far as I know), stability at high
temperatures when dehydrated, and the abundance of (highly incongruent) data on its solubility at different temperatures and hydration states, given
below from various internet sources:
| Quote: |
Wikipedia solubility table and Sciencemadness wiki
CuSO₄·5H₂O
231 g/L in water at 0°C
320 g/L in water at 20°C
1140 g/L in water at 100°C
Sciencemadness wiki
CuSO₄·0H₂O
143 g/L in water at 0°C
205 g/L in water at 20°C
754 g/L in water at 100°C
Sciencemadness wiki (physical properties desc.)
CuSO₄·5H₂O (assumed)
316 g/L in water at 0°C
2033 g/L in water at 100°C
Wikipedia page on copper sulphate
CuSO₄·0H₂O (assumed)
201 g/L in water at 20°C
PubChem source 1
CuSO₄·5H₂O (assumed)
243 g/L in water at 0°C
PubChem source 2
CuSO₄·0H₂O (assumed)
754 g/L in water at 100°C
PubChem source 3
CuSO₄·0H₂O (assumed)
203 g/L in water at 20°C
NPIC technical sheet
CuSO₄·0H₂O (pentahydrate stated, anhydrate assumed)
148 g/L in water at 0°C
736 g/L in water at 100°C
INCHEM safety data sheet
CuSO₄·5H₂O (assumed)
317 g/L in water at 0°C
Sigma Aldrich solubility table
CuSO₄·0H₂O (pentahydrate stated, anhydrate assumed)
148 g/L in water at 0°C
208 g/L in water at 20°C
736 g/L in water at 100°C
Sigma Aldrich solubility table
CuSO₄·5H₂O (anhydrate stated, pentahydrate assumed)
255 g/L in water at 0°C
362 g/L in water at 20°C
830 g/L in water at 100°C
Crystal growing wiki
CuSO₄·0H₂O
142 g/L in water at 0°C
200 g/L in water at 20°C
770 g/L in water at 100°C
Crystal growing wiki
CuSO₄·5H₂O
231 g/L in water at 0°C
326 g/L in water at 20°C
1150 g/L in water at 100°C
USDA data sheet
CuSO₄·5H₂O
316 g/L in water at 0°C
2033 g/L in water at 100°C
USDA date sheet
CuSO₄·5H₂O (anhydrate stated, pentahydrate assumed)
243 g/L in water at 0°C
320 g/L in water at 20°C
1140 g/L in water at 100°C
|
Of these 15 sources, 6 of them fail to specify the copper sulphate's hydration state and 4 of them appear to have swapped the data for the anhydrate
and pentahydrate (based on their dissonance with the other 11 sources).
The data from these sources is analyzed below.
| Quote: |
// list of all solubility data for the pentahydrate, and the average solubility
CuSO₄·5H₂O at 0°C 231 316 243 317 255 231 316 243 // 269 ± 43 g/L
CuSO₄·5H₂O at 20°C 320 362 326 320 // 332 ± 21 g/L
CuSO₄·5H₂O at 100°C 1140 2033 830 1150 2033 1140 // 1388 ± 602 g/L
-
// list of all solubility data for the anhydrate, and the average solubility
CuSO₄·0H₂O at 0°C 143 148 142 148 // 145 ± 3 g/L
CuSO₄·0H₂O at 20°C 205 201 208 200 // 204 ± 4 g/L
CuSO₄·0H₂O at 100°C 754 736 770 736 // 749 ± 17 g/L
// computed solubility for the pentahydrate based on the avg. solubility of the anhydrate
CuSO₄·5H₂O at 0°C 247 g/L // 8.9% below avg.
CuSO₄·5H₂O at 20°C 361 g/L // 8.7% above avg.
CuSO₄·5H₂O at 100°C 2030 g/L // 46.3% above avg.
-
// computed solubility for the anhydrate based on the avg. solubility of the pentahydrate
CuSO₄·0H₂O at 0°C 156 g/L // 7.6% above avg.
CuSO₄·0H₂O at 20°C 189 g/L // 7.9% below avg.
CuSO₄·0H₂O at 100°C 591 g/L // 26.8% below avg.
|
The solubility data for anhydrous copper sulphate is in very strong agreeance, with an average percent deviation of only around 2% between sources. By
contrast, the average percent deviation for the pentahydrate is over 20%.
The discrepancy for the pentahydrate solubility measurements at 100°C is especially strange, with an average deviation of 43% and values ranging from
2033 g/L down to 830 g/L.
However, what's interesting is that there are 2 sources that corroborate the computed solubility of 2030 g/L at 100°C for the pentahydrate almost
exactly, at 2033 g/L.
Judging by the huge range in the solubility values measured for the pentahydrate (particularly at 100°C), I suspect that the researchers had trouble
maintaining the copper sulphate at a stoichiometric pentahydrate.
I intend to test this, and try to come up with a reliable way to measure solubility values myself.
-
If there's errors in this post please let me know. Discussion is appreciated and I'd really like to get to the bottom of this, because it's a subject
that we should've had straightened out 200 years ago.
[Edited on 3/5/2023 by SnailsAttack]
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
A good protocol would be required - Lots of procedures.
Purification and analysis of reagents might be a collaborative first step?
An optional initial collaboration could be to choose one temperature (eg 50C) and members could submit their own results.
Each to their own capabilities with estimates of error.
I'd probly have a go at it..... if someone organised it 
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Hi SnailsAttack, good to see the continuation of this after your last thread. I agree with Sulaiman sentiments and would be happy to contribute, if
you decide you would like experimental input from other members.
|
|
|
Rainwater
National Hazard
   
Posts: 937
Registered: 22-12-2021
Member Is Offline
Mood: Break'n glass & kick'n a's
|
|
If you can write a step-by-step, I'm in.
I've always assumed that the solubility of a salt is equal to a molar ratio with water for a given temperature.
"You can't do that" - challenge accepted
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Just some ideas
Someone (if no volunteer then I'll volunteer, if before May) could provide, via post,
'reference samples' for those interested,
maybe in return for p&p and misc. costs.
This would enable a degree of cross-checking our own results.
Ideally the samples should be of high purity etc.
But shared 'reference samples' of lower quality may serve the same purpose. (?)
I'm thinking small packets (e.g. 10g?) just to be used for 'calibration'
but if postal rates are acceptable then possibly enough for all requirements ?
P.S. I may be biased because I too expect the saturated concentration of ions will be independent of whether the anhydride or pentahydrate is
used.
If any other result then, I would first suspect my own procedures 
but... who KNOWS ?
For the initial collaboration it may be better for each participant to measure solubility at their local room temperature
Just for practical reasons.
[Edited on 6-3-2023 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
It's actually quite difficult to measure solubility accurately (especially far from room temperature).
How accurate an answer do you actually ever need?
If you use molarity, the hydration doesn't matter.
|
|
|
teodor
National Hazard
   
Posts: 923
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
Near 95C is the crytical point after which water solution gives CuSO4*3H2O crystalls. And below this point probably it would be mixture of penta- and
tri- hydrate. That's why solubility data at high temperature can disagree with each other.
Another challenge is hydrolisis to basic copper sulfate, I guess for this reason some authors can measure solubility of CuSO4 in acidic solution and
interpolate the results.
If you define the solubility as an "ammount of solid which can be dissolved" it's better to use the data for anhydrous sulfate.
As I already mentioned in the previous thread, the classical definition is equillibrium, so it depends also on crystallisation properties.
Also, it should be mentioned that "amount of solid which can be dissolved" in the real life is never possible to dissolve without either raising the
temperature or adding some additional water, that's why it is never used as a definition.
[Edited on 6-3-2023 by teodor]
[Edited on 6-3-2023 by teodor]
|
|
|
yobbo II
National Hazard
   
Posts: 764
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
There is also the question of the meta stable state. When you are dissolving (or crystallizing) you must wait for quite a long time for ALL
of the stuff that can go into solution (or come out) to go into solution at the given temperature. Most studies use a temperature controlled set up
and stirr for 24 hours.
No solvent must be let escape as well.
Then there is the problem of when you extract the crystals, ( either extra crystals of stuff that you have put there to allow saturation to occur (or
crystals that have come out of solution, if you are crystallizing)) the water stuck to these crystals will have a certain weigh and will also have a
certain amount of the stuff dissolved in it. How do you measure this?
The method of wet residues is used (there may be other methods) to measure this liquid.
See https://pubs.acs.org/doi/10.1021/je00103a002
read the first few lines.
What I am trying to say is that it is quite a task to measure the whole thing accurately.
The higher the measuring temperature the more the hassel. You need preheated filters, etc etc
With very soluble (not CuSO4) compounds the whole thing is a nightmare. Some substances will dissolve in their own water of crystallization.
Yob
|
|
|
Texium
Administrator
       
Posts: 4618
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
As the last three posters have pointed out, solubility is not as easy to measure as one would think. I’d also argue that it’s hardly important.
The only time I look up solubility data for a compound is if I’m making a saturated solution of some common salt to keep on hand, and even then I
overshoot by a few grams/L to ensure that it will stay saturated with potential temperature fluctuations. So what if there’s some undissolved solid
at the bottom of the bottle.
So my question is, why even bother? I won’t accept “for fun” as an answer, because frankly there is nothing fun about measuring the solubility
of salts to a high degree of accuracy, and I can guarantee that there are much more fulfilling ways you could be spending your precious lab time!
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Good suggestion. I've found that some salts which exhibit hydration behavior behave very nicely while others are a complete mess.
Quote: Originally posted by Sulaiman  | Maybe decompose and compare to copper oxide weight if >650C available,
or (zinc or Iron etc.?) precipitation of copper, dry, weigh etc.
Maybe Iodometric titration for greater accuracy.
Something like that. |
I probably can't maintain a sample at 650°C+ on the stovetop but I could convert the copper sulphate to copper oxyhydroxide and then pyrolyze that
and weigh it.
Quote: Originally posted by Sulaiman  | Maybe a first calculation would be what accuracy of results is required?
For example, in absolute terms, I can weigh to great accuracy,
but volumes are only accurate to about +/- 0.1%, on a good day
and temperature accuracy is even worse at about +/- 1C. |
Solubility measurements extrapolated from individual mass measurements rather than volume measurements would be ideal, yes.
According to this graph (which appears to be for the anhydrate), a difference of 1°C at 20°C affects the solubility by 3.5 g/L, equivalent to an error of
1.73%.
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Quote: Originally posted by Texium  | As the last three posters have pointed out, solubility is not as easy to measure as one would think. I’d also argue that it’s hardly important.
The only time I look up solubility data for a compound is if I’m making a saturated solution of some common salt to keep on hand, and even then I
overshoot by a few grams/L to ensure that it will stay saturated with potential temperature fluctuations. So what if there’s some undissolved solid
at the bottom of the bottle.
So my question is, why even bother? I won’t accept “for fun” as an answer, because frankly there is nothing fun about measuring the solubility
of salts to a high degree of accuracy, and I can guarantee that there are much more fulfilling ways you could be spending your precious lab time!
|
The 'why even bother' is that there still seems to be an unexplained phenomenon here and just because three posters have said solubility is hard to
measure is not a good reason to not undertake an experiment. I do not want to be putting words in the mouth of the OP so please correct me if I am
wrong, but this is not about being precise when making up a saturated solution.
In all of the posts on this thread and the other relating to this matter, the observed (based on published solubilities) difference in solubility
between anhydrous and hydrated salts (taking into account the additional water in the hydrate) has still not been explained.
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
The first step to this is to see whether copper sulphate actually crystallizes from solution to form an ideal stoichiometric pentahydrate.
  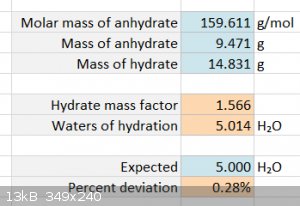
CuSO₄·5H₂O(s) -> CuSO₄(s) + 5H₂O(v)
Incredibly, it does, it behaves perfectly. I pyrolyzed ~15 grams of hydrated copper sulphate and measured a mass reduction of 0.6386x compared to the
theoretical of 0.6392x. That's an error of 0.10%.
The anhydrous copper sulphate seems to be very hygroscopic, and pulls water from the air on standing. I'm not sure what hydration state it tends to.
Doesn't matter for the purpose of this thread.
|
|
|
Texium
Administrator
       
Posts: 4618
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by B(a)P  | The 'why even bother' is that there still seems to be an unexplained phenomenon here and just because three posters have said solubility is hard to
measure is not a good reason to not undertake an experiment. I do not want to be putting words in the mouth of the OP so please correct me if I am
wrong, but this is not about being precise when making up a saturated solution.
In all of the posts on this thread and the other relating to this matter, the observed (based on published solubilities) difference in solubility
between anhydrous and hydrated salts (taking into account the additional water in the hydrate) has still not been explained. |
A discrepancy between different data sets is not an “unexplained phenomenon” it just means that the result can be affected by a
multitude of variables, and controlling one may make it nearly impossible to control others. I have read the other thread, and I think teodor’s
comment that solubility is a state of equilibrium is important to highlight again. We like to talk about the solubility of a given substance at a
given temperature as though it is an immutable physical constant, but it is not. Some salts behave better than others, but it’s always an
equilibrium, and even if you use a very consistent method, you’re bound to get slightly different results sometimes. With salts that can form
multiple hydrates, have a tendency to supersaturate, and/or are deliquescent (e.g. sodium sulfate, sodium acetate, calcium chloride) calculating a
meaningful value becomes even more futile, hence the severe discrepancies highlighted for those in the other thread.
To be clear, I’m not trying to discourage experimentation on this simply because it’s hard to do, but because it’s very hard to do in a way that
will actually yield meaningful results. What is the best outcome from this? Another result that may agree partially with some of the existing
measurements, or possibly none of them at all? I don’t think the CRC would be rushing to revise their handbooks.
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Quote: Originally posted by Texium  | | We like to talk about the solubility of a given substance at a given temperature as though it is an immutable physical constant, but it is not.
|
Really?
Other than the chemical composition and the environment of our hypothetical copper sulphate solutions,
what could cause the equilibrium to not be a constant ?
(this is precisely what the OP question implies)
Not trying to rewrite the CRC handbook,
just settle the copper sulphate anhydrous vs pentahydrate anomalous (or not) solubility question.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by Texium  | | As the last three posters have pointed out, solubility is not as easy to measure as one would think. I’d also argue that it’s hardly important.
|
Solubility might be difficult to measure, but I think ballpark-accuracy solubility data is valuable to have.
Quote: Originally posted by Texium  | | The only time I look up solubility data for a compound is if I’m making a saturated solution of some common salt to keep on hand, and even then I
overshoot by a few grams/L to ensure that it will stay saturated with potential temperature fluctuations. So what if there’s some undissolved solid
at the bottom of the bottle. |
Online measurements of the solubility of copper sulphate at room temperature range anywhere from 200 g/L to 320 g/L. A solution in that range of
accuracy is fine for analytical chemistry, but if you knew the exact solubility you could infer the molarity of a saturated solution, which could be
really useful.
Quote: Originally posted by Texium  | | So my question is, why even bother? I won’t accept “for fun” as an answer, because frankly there is nothing fun about measuring the solubility
of salts to a high degree of accuracy, and I can guarantee that there are much more fulfilling ways you could be spending your precious lab time!
|
No yeah I completely agree, this is super boring. B(a)P describes my exact reason for taking interest nonetheless:
Quote: Originally posted by B(a)P  | The 'why even bother' is that there still seems to be an unexplained phenomenon here and just because three posters have said solubility is hard to
measure is not a good reason to not undertake an experiment.
In all of the posts on this thread and the other relating to this matter, the observed (based on published solubilities) difference in solubility
between anhydrous and hydrated salts (taking into account the additional water in the hydrate) has still not been explained. |
Other observed phenomena include:
- Woelen's claim that vanadyl sulphate is virtually insoluble in water while its hydrate dissolves readily. I'd like to test this myself with the
vanadyl you sent me.
- Various strange behavior concerning the solubility of hydrated sodium sulphates discussed by Teodor and Tsjerk.
- B(a)P noted that online solubility measurements for anhydrous sodium sulphate at 20°C range anywhere from 139 to 445 g/L.
- According to wikipedia, anhydrous sodium acetate is ~3x more soluble in water than the trihydrate, but the data in this post suggests that the
hydrated salt should always be more soluble.
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by Texium  | | A discrepancy between different data sets is not an “unexplained phenomenon” it just means that the result can be affected by a multitude of
variables, and controlling one may make it nearly impossible to control others. |
Quote: Originally posted by Texium  | | I think teodor’s comment that solubility is a state of equilibrium is important to highlight again. We like to talk about the solubility of a given
substance at a given temperature as though it is an immutable physical constant, but it is not. |
There can't be that many variables, can there? i would think that the hydration state of emerging salts and their equilibrium with the solution would
be controlled solely by the temperature.
Quote: Originally posted by Texium  | | With salts that can form multiple hydrates, have a tendency to supersaturate, and/or are deliquescent (e.g. sodium sulfate, sodium acetate, calcium
chloride) calculating a meaningful value becomes even more futile, hence the severe discrepancies highlighted for those in the other thread.
|
Depending on how the solubility measurements are performed, supersaturation and deliquescence aren't necessarily a problem. The main showstopper would
be the salts that evaporate down to a goo instead of crystallizing (e.g. magnesium acetate, nickel acetate), since the line between "solid" and
"liquid" is indistinguishable.
Quote: Originally posted by Texium  | | What is the best outcome from this? Another result that may agree partially with some of the existing measurements, or possibly none of them at all? I
don’t think the CRC would be rushing to revise their handbooks. |
not to be conceited, but I trust our observations over the institutional ones
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by Sulaiman  | A good protocol would be required - Lots of procedures.
Purification and analysis of reagents might be a collaborative first step?
An optional initial collaboration could be to choose one temperature (eg 50C) and members could submit their own results.
Each to their own capabilities with estimates of error.
I'd probly have a go at it..... if someone organised it  |
Quote: Originally posted by B(a)P  | | Hi SnailsAttack, good to see the continuation of this after your last thread. I agree with Sulaiman sentiments and would be happy to contribute, if
you decide you would like experimental input from other members. |
Glad to hear you guys are interested. I'm down for organizing a collaboration on solubility measurements once I've got a reliable procedure figured
out.
Quote: Originally posted by Sulaiman  | Someone (if no volunteer then I'll volunteer, if before May) could provide, via post,
'reference samples' for those interested,
maybe in return for p&p and misc. costs.
This would enable a degree of cross-checking our own results.
Ideally the samples should be of high purity etc.
But shared 'reference samples' of lower quality may serve the same purpose. (?)
I'm thinking small packets (e.g. 10g?) just to be used for 'calibration'
but if postal rates are acceptable then possibly enough for all requirements ? |
I've only got like 20 grams of copper sulphate but I have a whole bunch of sodium acetate and magnesium sulphate I could send that I think would
behave well for solubility measurements.
Working on it.
|
|
|
teodor
National Hazard
   
Posts: 923
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
Quote: Originally posted by Sulaiman  |
Other than the chemical composition and the environment of our hypothetical copper sulphate solutions,
what could cause the equilibrium to not be a constant ?
|
It could not ever exist, like equilibrium between CuSO4 (anhydrous) crystalls and water under 100C or CuSO4 * 5H2O and water above 96C.
But I have my own interest to compose the table of solubility of salts of mono- and di- carboxylic acids with different metals up to C6, so I will be
happy to read the experiment results and will hope it will be possible to extend your method for measuring those solubilities which are probably
completely unknown.
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
This is, uhh, pretty silly. Examine solubility from the perspective of crystallizing a compound. It’s all to common to encounter supersaturation of
a solution, and in general it’s an incredibly hard-to-predict process. Then examine every instance of a YouTube video where more than the
theoretical amount of water or another solvent is needed to dissolve something. Then look at how incredibly slow further dissolution is as you
approach the solubility limit of a given compound in a given volume of solvent. It’s all very cryptic and inconsistent. There are far too many
variables at play, clearly many that we don’t even know or have words to describe, as this thread has pointed out. You’re not going to crack the
case in a home lab with reagents you cannot properly purify or analyze, in a non-controlled setting for variables like temperature, humidity, etc.
This isn’t a problem that needs solving and it’s not one you won’t figure it out standing at a lab bench with basic equipment.
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
please watch me nail this entire project
|
|
|
yobbo II
National Hazard
   
Posts: 764
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
If there is a difference in solubility between the Cu and SO4 ion pair that comes from anhydrous Copper Sulphate and the ion pair that comes from the
hexahydrate (or whatever hydrate) then the water has some way of 'distinguishing' between the different source of ion pairs.
This is impossible? (surley).
One 'set' from a particular source may dissolve more quickly that the other but if given enough time both must have equal solubility.
Copper sulphate has hydrates from 1 to 7 according to the net.
The solubility according to Seidell is below.
Perhaps this data is included in the first post.
Yob

Attachment: dehyd_OF_cu_sulfate.pdf (1.8MB)
This file has been downloaded 239 times
|
|
|
Texium
Administrator
       
Posts: 4618
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by yobbo II  | | One 'set' from a particular source may dissolve more quickly that the other but if given enough time both must have equal solubility.
|
Yes.
I was typing out a longer response last night, but I lost it by accident, and teodor and Amos pretty much covered what I was going to say.
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Help prevent my brain from breaking!
What would happen if anhydrous copper sulphate is added to an already saturated solution?
Meanwhile....
I think that Iodometric titrations will be required to measure copper sulphate concentrations.
Or is there a better (cheaper, easier, more accurate etc.) method.
[Edited on 8-3-2023 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Texium
Administrator
       
Posts: 4618
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Good luck bud
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|

Quote: Originally posted by teodor  | | Near 95C is the crytical point after which water solution gives CuSO4*3H2O crystalls. And below this point probably it would be mixture of penta- and
tri- hydrate. That's why solubility data at high temperature can disagree with each other. |
Maybe. Could be tough to maintain a 100°C bath anyways.
Quote: Originally posted by teodor  | | Another challenge is hydrolisis to basic copper sulfate, I guess for this reason some authors can measure solubility of CuSO4 in acidic solution and
interpolate the results. |
Noted.
Quote: Originally posted by yobbo II  |
There is also the question of the meta stable state. When you are dissolving (or crystallizing) you must wait for quite a long time for ALL
of the stuff that can go into solution (or come out) to go into solution at the given temperature. Most studies use a temperature controlled set up
and stirr for 24 hours. |
24 hours? I give it five minutes max before a sample of solid copper sulphate emersed in water is effectively fully equilibriated with the solution.
Quote: Originally posted by yobbo II  | Then there is the problem of when you extract the crystals, ( either extra crystals of stuff that you have put there to allow saturation to occur (or
crystals that have come out of solution, if you are crystallizing)) the water stuck to these crystals will have a certain weigh and will also have a
certain amount of the stuff dissolved in it. How do you measure this?
The method of wet residues is used (there may be other methods) to measure this liquid.
See https://pubs.acs.org/doi/10.1021/je00103a002
read the first few lines. |
... how do you envision the measurement process to work? I guarantee it doesn't require supping water off the crystals and using a mathematic model to
calculate how much is left on them.
|
|
|
| Pages:
1
2
3
4 |