Hey Buddy
Hazard to Others
  
Posts: 441
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Are pyrroles any good for EM building blocks?
I've had this paper kicking around in my brain for about 5 years now. I never hear about pyrroles. Is that because they arent much use? Or because
they are just unexplored. See image. sorbic acid and nitrite gives a methyl-pyrrole with two nitro groups already there. It just always struck me as
odd. I wonder how useful that could be building something else off of it? Or if theres another acid that would respond even better to nitrite
treatment.
Really, I ordered a big bucket of nitrite. So i'm on a theoretical nitrite bender.

[Edited on 19-11-2022 by Hey Buddy]
My first thought of potential when I saw the paper was thinking back to dpx-1 "non-traditional explosiphore" klapotke paper. Obviously a furoxan isn't
a pyrrole, but I would never assume the Klapotke molecule would detonate @ 8245m/s 29GPa. I'm just curious if anyone knows anything about pyrroles
before I start getting all mad-max lab in the old garage.
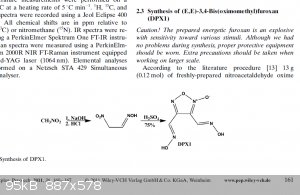
[Edited on 19-11-2022 by Hey Buddy]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Hey Buddy  | I've had this paper kicking around in my brain for about 5 years now. I never hear about pyrroles. Is that because they arent much use? Or because
they are just unexplored. See image. sorbic acid and nitrite gives a methyl-pyrrole with two nitro groups already there. It just always struck me as
odd. I wonder how useful that could be building something else off of it? Or if theres another acid that would respond even better to nitrite
treatment.
Really, I ordered a big bucket of nitrite. So i'm on a theoretical nitrite bender.
My first thought of potential when I saw the paper was thinking back to dpx-1 "non-traditional explosiphore" klapotke paper. Obviously a furoxan isn't
a pyrrole, but I would never assume the Klapotke molecule would detonate @ 8245m/s 29GPa. I'm just curious if anyone knows anything about pyrroles
before I start getting all mad-max lab in the old garage.
[Edited on 19-11-2022 by Hey Buddy] |
Those two examples of unusual- unconventional energetic materials are of course very interesting... but brings also into light the extreme
complexity/versatility and possibilities of the HNO2 / NO(+) chemistry.
The heterocyclic chemistry is also quite wild and full of specific reactions depending onto the hetero atoms, their type, their electronegativity,
their numbers and the insaturations into the cycle leading sometimes to aromaticity with help of heteroatoms non-binding doublets...
A simple comparison of pyrrole, furan and thiophene give you a hint of the effect of a single heteroatom change into a molecular structure...also the
effect of increasing the number of heteroatoms of a single species like N by passing from a single N into pyrrole, to 2Ns, 3Ns or tetrazoles.
The cyclic penta-rings are of course of great interest into the EM world because of the compacity of the molecule...5 atomic cyclic ring members
offers structurally some stability and compactness ... so density increase and less sensitivity/more stability.
Into the EM/HEM world... you often find pentaring energetic groups (like tertrazoles, furazans, furoxans, oxadiazoles, triazoles.
Heteroatoms often increase the density vs a conventionnal -CH= or -CH2-part because for an equivalent or near equivalent volume CH or CH2 weights
13-14g vs 14g for -N= or 16g for -O- or even 32g for -S-
The main problem is the very exotic/specific chemistry of such cyclic compounds and heteroatoms so variable from what we know and that change from
position relatative to each other and position into the ring...
So you cannot easily put as much of what you want where you want to...also each group will change the properties of the ring and of the heteroatom but
also the reactivity of each group already in place... it becomes a complex cards castle sensitive to wind and rain...
Thus OK two NO2 is better than one but 3,4 or 5 can seriously generate unstability or lead to inexistance...
See already hexanitrobenzene... that is difficult to get at that hexa-nitro level...it is powerful but unstable thermally and hydrolytically (turns
into trinitrophloroglucidol - 1,3,5-trihydroxy-2,4,6-trinitrobenzene).
About your nitrite bending ("the last NO bender?" 
I think that to buy and store a quantity of NaNO2 is a good idea... because general chemistry of NO(+), NO, HONO is really interesting and especially
for some EnM... I will try to give hereafter... a tiny tour of those possibilities by working a bit onto your examples and other known EnM 
[Edited on 22-1-2023 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 441
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Here in the USA I purchase NaNO2 from Duda Energy out of Alabama. I had a big order of NaNO2 from them at the time of this first post. They had
a reallocation of nitrite and cancelled the order and removed nitrite from their catalogue. I talked to them on the phone, they expect to carry it
again someday but dont know when. (I suspect it is all going to war). --Anyways, I realized after this loss of nitrite availability, without nitrite
there are brakes put on your experimenting and testing -No azides, tetrazoles, DDNP. A challenge. I dont know of any clean high yielding reductions of
NO3 to NO2 but I may try different reductions just to try and learn about the possibilities. If you can get NaNO2 take advantage of the availability.
If Duda produces it again I will just buy the 50lb bag and pay freight next time for lifetime supply.
|
|
|
Microtek
National Hazard
   
Posts: 877
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Yes, it is annoying when the powers that be block access to certain chemicals. Here in Europe, things such as nitrite, HNO3 above 3% conc and H2SO4
above 50% conc are banned. It forces you to think creatively.
|
|
|
underground
National Hazard
   
Posts: 708
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Quote: Originally posted by Hey Buddy  | Here in the USA I purchase NaNO2 from Duda Energy out of Alabama. I had a big order of NaNO2 from them at the time of this first post. They had
a reallocation of nitrite and cancelled the order and removed nitrite from their catalogue. I talked to them on the phone, they expect to carry it
again someday but dont know when. (I suspect it is all going to war). --Anyways, I realized after this loss of nitrite availability, without nitrite
there are brakes put on your experimenting and testing -No azides, tetrazoles, DDNP. A challenge. I dont know of any clean high yielding reductions of
NO3 to NO2 but I may try different reductions just to try and learn about the possibilities. If you can get NaNO2 take advantage of the availability.
If Duda produces it again I will just buy the 50lb bag and pay freight next time for lifetime supply. |
https://www.youtube.com/watch?v=tMltPEUNgSM
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
NaNO2 isn't to hard to produce- And it is available OTC anywhere they make saussage and ham. Look for "curing salt".
https://youtu.be/wIPrEZgegck
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Microtek
National Hazard
   
Posts: 877
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Yes, but nitrates are also restricted. The only remaining source that I'm aware of is calcium ammonium nitrate. The curing salt that you can buy
around here (as a private citizen) contains 0.4 % sodium nitrite, the balance being sodium chloride.
|
|
|