Chris The Great
Hazard to Others
  
Posts: 463
Registered: 29-10-2004
Location: Canada
Member Is Offline
Mood: No Mood
|
|
Nitramide reactions
Some ideas occured to me recently, about using nitramide to prepare interesting and powerful energetic materials.
I haven't been able to find much information to confirm whether my ideas would or would not work however, except the abstract of a journal (see
below).
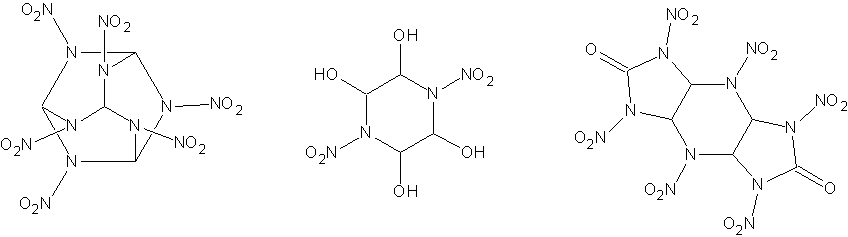
The first idea was to synthesis HANA (hexaazahexanitroadamantine), C4H4N12O12, which has a density of 2.10 g/cc. In this case the reaction would be:
6NH2-NO2 + 4CHI3 -> C4H4N12O12 + 12HI
The HI would most likely have to be absorbed by a base, ie triethylamine, that will not decompose nitramide rapidly. I would think the high
reactivity of the C-I bonds would allow this reaction to take place readily. On the other hand the probable strain of the molecule would make the
reaction disfavourable. They might also react to form other products, and not form HANA at all.
So, I am not really sure whether that would work, if it did it would be quite easy to do at home and would produce a very interesting energetic
material.
The second one would be reacting nitramide with glyoxal, to form a piperidine ring NO2-N(CH(OH)-CH(OH))2N-NO2. This would no doubt lend itself well
to the synthesis of some interesting energetics.
The original compound I had in mind that could be made from it was HHTDD, which would be formed by condensing the above compound with dinitrourea,
adding a dinitrourea group on each side and eliminating water. Since water would decompose the DNU, would would have to form the tetracetyl of the
nitramide product, and react that with the DNU instead as acetic acid would form, and it will not hydrolysis the DNU.
HHTDD has a densitry of 2.07 g/cc, perfect O balance, and is considered to be the most powerful explosive known. However, the nitration conditions
require large amounts of 100% HNO3 and N2O5 and so straight synthesis would be difficult.
If nitramide forms the wanted ring, then the only starting materials are acetic anhydride, dinitrourea, glyoxal and the appropriate solvent, and maybe
a catalyst.
The evidence I have for nitramide reacting with aldehydes comes from a journal abstract I came across while searching to see if these ideas would
work, in which nitramide and formaldehyde formed a stable and powerful polymer.
A Study on the Condensation of Nitramide (NH2NO2) with Formaldehyde and the Characteristics of the Products Formed
Z. Raszewski, M. Syczewski
Propellants, Explosives, Pyrotechnics
Volume 24, Issue 6 , Pages 366 - 370
It would be nice if someone got it, the polymer looks like it would be interesting on it's own.
(CH2N-NO2)n --> nCO + nH2O + nN2
I have attached drawings of HANA, NO2-N(CH(OH)-CH(OH))2N-NO2 (not sure of the name) and HHTDD so it is much clearer what I am talking about.
Thoughts?
|
|
|
tom haggen
Hazard to Others
  
Posts: 488
Registered: 29-11-2003
Location: PNW
Member Is Offline
Mood: a better mood
|
|
That is a massive amount of gass molcules
N/A
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Nice ideas
Your HANA, do you have a reference on this?
Iodoform and nitramine - sounds simple enough. Do you have decent references on the production of H2N-NO2? I thought it was notoriously unstable.
I imagine getting the final product would be difficult as the proportions have to be gotten exact. How would you avoid getting intermolecular
crosslinks, rather than this caged structure?
The reaction of the C-I moiety with H-NR2 would require conditions generally used for N-alkylations (similar to what was asked in the novocaine
thread).
On the second one, I guess you got the idea from the tetranitropiperazine, which was described in one of the PEP papers by this Munich group (Klapoetke)?
I suppose this could work, they use formamide as opposed to nitramide.
Nitrating this is gonna be a pain or two, nonetheless (TFAA and such).
Re. these polymers - I had similar ideas like that before. For instance, I condensed CH2O with guanidine BASE, and very soon after, a white plasticky
substance formed.
Of course this could now be modified to use nitroguanidine, nitro urea, and so on, and possibly make the nitrate salt of this afterwards.
Alternatively one could start playing with semicarbazide, or aminoguanidine, and so on. So many things to do!
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Chris The Great
Hazard to Others
  
Posts: 463
Registered: 29-10-2004
Location: Canada
Member Is Offline
Mood: No Mood
|
|
No reference on the HANA, just popped into my head one day. So I have no clue if it will actually produce the wanted product, though I am sure they
will react to produce high nitrogen content compounds.
Second one also popped into my head, though nitrating to the tetranitro was inspired by that. But the main idea behind it would be an easy production
of HHTDD without having to go through a difficult nitration process, which seems to be the main problem behind making it. I read about HHTDD long
before I heard that the precursor could also be nitrated to a powerful explosive as well. It them occured to me that since the end nitrogens on the
molecule are the ones the require the difficult nitration conditions, it would be possible to condense with dinitrourea rather than urea and remove
the need for the harsh nitration conditions. Then, I thought of using nitramide instead of formamide, and not needing any nitration at all. Seemed
like something worth sharing.
Nitramide is produced from hydrolysis of dinitrourea, which proceeds immediatly upon addition to water. I am not sure what yields are after
extracting, although it may not even be necessary and the solution of nitramide formed might be possible to use directly in the procedures.
It fits well with using it to form HHTDD, since it will be formed from DNU, and then DNU is used again to make the HHTDD. Dinitrourea is made by a
straightforward nitration of urea, however it is very impure and rapidly breaks down if not washed with trifluoroacetic acid after the nitration
(other solvents probably work but haven't been revealed yet). Yields are around 75% or so, and it may not need to be washed if it is just
hydrolysed after the synthesis to make nitramide.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Definatly interesting, I think the HANA reaction is pretty likely if the C-I with H-N is able to take place in the standard way. There is actually
very little strain in this molecule, after all it is (figurativly) a bunch of cyclohexane rings. Since cyclohexane pretty much has a strain of zero,
the strain on this molecule is only slightly greater. I made this molecule with a molecular kit and there was only very mild forcing of the last bond
together(unlike cubane which snapped the sp3 carbon when making the molecule).
Compared with what else could theoretically form based on the reaction of just the C-I and H-N, such as cyclobutane rings in the same format as the
adamantane structure, and cyclononane based structures, and even larger, ring strain reaallly favours the adamantane structure.
Hehe...use iodopicrin instead of iodoform,   ....4 more nitros on the molecule ....4 more nitros on the molecule
|
|
|
Axt
National Hazard
   
Posts: 794
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Chris The Great
A Study on the Condensation of Nitramide (NH2NO2) with Formaldehyde and the Characteristics of the Products Formed
Z. Raszewski, M. Syczewski
Propellants, Explosives, Pyrotechnics
Volume 24, Issue 6 , Pages 366 - 370
|
For whatever reason I found that on my desktop, forget what I was thinking when I got it. I put one on nitrourea -> nitramide in the zip as well.
I think anyone can open PEP articles from between 1998-2002 through wiley, and they are all downloadable on one of the ftp's.
http://www3.interscience.wiley.com/cgi-bin/fulltext/5008392/...
That opens ... right? click the "PDF" link.
[Edited on 27-11-2005 by Axt]
Attachment: nitramide.zip (848kB)
This file has been downloaded 989 times
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
1)Exchange hability of iodine from CHI3 is much lower than from CI3-NO2....To play with NH2-NO2 is a good idea but in the hypothetical point of view
HI will be formed..HI is strong acid and reducer...so chances of NH2-NO2 to survive which are already low will be even lower....but who knows...trial
doesnt cost much...
To overcome this HI problem ... it must be possible to use Ag-NH-NO2 in a way to drive the reaction to the good side without the annoying acid
effect...
O2N-CI3 + 3 Ag-NH-NO2 --> O2N-C(NH-NO2)3 + 3AgI...
Being bounded so the resulting molecule (maybe very prompt to hydrolyse) must be a very strong acid...
So silver salt might be formed a second time...
O2N-C(NH-NO2)3 +3/2 Ag2CO3--> O2N-C(AgN-NO2)3 + 3/2 CO2 + 3/2 H2O
The later then reacted in the same fashion as the first time will give plannar polymer or the caged adamantane sphere you imagined but with nitros on
each top...what is in favour of the spherisation or of the plannification of your multimeric coupound.
2)The second molecule you named HHTDD is indeed powerful and many trials have proven the cyclisation between O2N-N(CHOH-CHOH)2N-NO2 and DNU is very
hard owing to the fact the two HO points in different directions...so it is very hard to get them to react 2 by two to get a first stressed pentaring
and then a second one.
Thus the presence of the NO2 on the initial core molecule is a disavantage since they tends to improve chair shape instead of the boat you must have
to allow easier cyclisation.
One possibility but I don't see how to get there...is to start from:
O2N-N(CI=CI)2N-NO2 (planar with I external in the plane)+2 (AgN(NO2))2C=O....you then possibly end up with the squeletton you asked but with two more
insaturations...and nearly perfect planarity....
Another possibility would be to bend the initial molecule by bridging the two external Ns...bridge must be extractible once reaction is achieved
without destroying the two others made rings...and also exchanged for NO2...
[Edited on 20-12-2005 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Chris The Great
Hazard to Others
  
Posts: 463
Registered: 29-10-2004
Location: Canada
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by PHILOU Zrealone
2)The second molecule you named HHTDD is indeed powerful and many trials have proven the cyclisation between O2N-N(CHOH-CHOH)2N-NO2 and DNU is very
hard owing to the fact the two HO points in different directions...so it is very hard to get them to react 2 by two to get a first stressed pentaring
and then a second one.
Thus the presence of the NO2 on the initial core molecule is a disavantage since they tends to improve chair shape instead of the boat you must have
to allow easier cyclisation. |
Hmm, what are the sources here? I'm curious because the same molecule (with CHO instead of NO2) reacts readily with urea to form the wanted molecule,
so I expected the reaction with DNU would also occur easily.
You are correct about HI reducing the NO2 groups, I didn't think about it but it is a possible side reaction that could occur quite easily and destroy
the final product. Would it still happen if the HI is removed by a base? I would imagine that the reaction would at least be reduced drastically,
but unfortunately it adds cost and complexity to the reaction.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
| Quote: | Originally posted by Chris The Great
Hmm, what are the sources here? I'm curious because the same molecule (with CHO instead of NO2) reacts readily with urea to form the wanted molecule,
so I expected the reaction with DNU would also occur easily.
|
 Unfortunately I don't have readily acces to my books and notes(everything is in
cardboard boxes for two years now-new mother's appartement, buildingwork, and now I go live with my girlfriend )...maybe in two weeks I wil be able to
answer your question... Unfortunately I don't have readily acces to my books and notes(everything is in
cardboard boxes for two years now-new mother's appartement, buildingwork, and now I go live with my girlfriend )...maybe in two weeks I wil be able to
answer your question...
Regarding your -CH=O instead NO2...I wonder how do you account for the fact urea doesn't react with -CH=O?
| Quote: | Originally posted by Chris The Great
You are correct about HI reducing the NO2 groups, I didn't think about it but it is a possible side reaction that could occur quite easily and destroy
the final product. Would it still happen if the HI is removed by a base? I would imagine that the reaction would at least be reduced drastically,
but unfortunately it adds cost and complexity to the reaction. |
I guess this would be a solution...the base would activate the exchange of I...by scavenging the HI as soon as it forms...Of course the use of
nitramine salts...is precisely a bit the same idea but under another form...
NH2-NO2 + OH(-) --> NH(-)-NO2 + H2O
CH3-I + NH(-)-NO2 --> CH3-NH-NO2 + I(-)
So if you also use the precipitation as a guiding line...
Silver is used as typical example for nucleophilic substitution of I but it is possible with other metals...
AgNH2-NO2 + CH3-I --> CH3-NH-NO2 + AgI
Also salts of nitramides are chemically stabler than the parent nitramide with regards to hydrolysis...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Chris The Great
Hazard to Others
  
Posts: 463
Registered: 29-10-2004
Location: Canada
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by PHILOU Zrealone
Regarding your -CH=O instead NO2...I wonder how do you account for the fact urea doesn't react with -CH=O? |
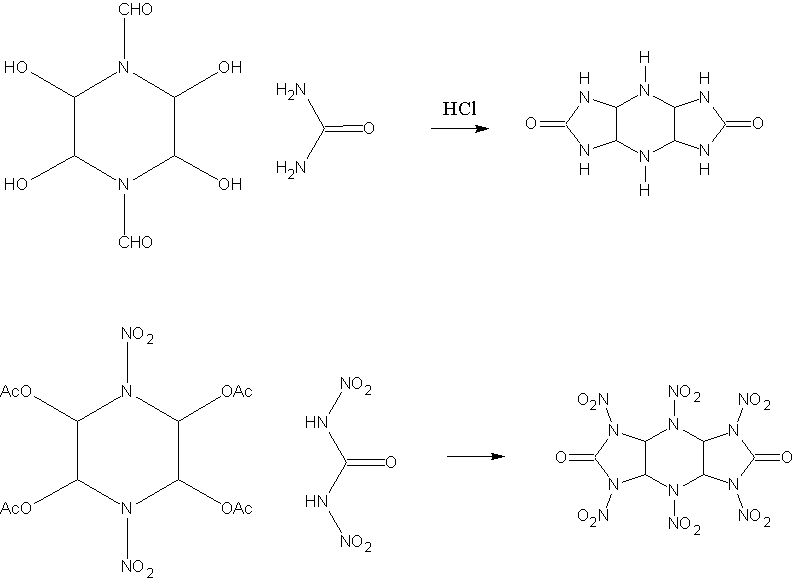
You are misunderstanding what I have in mind. I've attached a diagram of what reaction works, and what I have proposed using DNU. OAc is used
because otherwise the water produced by the reaction would destroy the DNU. In the first reaction, the CHO is hydrolysed during the reaction.
It may need other incentives to get the reaction to proceed fully however, ie basic enviroment etc.
|
|
|
simply RED
Hazard to Others
  
Posts: 209
Registered: 18-8-2005
Location: noitacoL
Member Is Offline
Mood: booM
|
|
greetings Philou
The second reaction in the last attachment is possible. One majour drawback, the first molecule (tetraacetyl dinitro derivative) is extremely hard to
synthese.
The existance of AgNH-NO2 is under question.
"...trial doesnt cost much..."
Oh, it does...
High-tech laboratory complex is needed to experiment with cyclic nitramines. NMR and IR are out of question. 100% HNO3 is pain in the ass (the toxic
effects on the lungs may be hidden for weeks !!!!!!!!!!!!!!!!!!!!!!!!).
I thought a "normal" fume hood will save me from NOx when working with liters HNO3... Wrong - you can smell it! A gas mask is also needed for full
protection.
(accessible antidots for H(NOx) - immediately - ammonia (carefully breathe 3 times - 10seconds each) , second vitamine C (500mg immediately - 3x200mg
per day - 5 days, then 3x100mg 20 days (from a military tox book)
P2O5 mixed with HNO3 is another trouble , igniting everything it falls on. Acetic anhydride mixed with c.HNO3 is a high explosive on itself (it could
be high, primary, or just burn - depending on accidental conditions) .
There is no problem to make - medina for example - in BAD equipped lab, but more complex thing is very problematic.
When logic and proportion have fallen sloppy dead...
|
|
|
Madandcrazy
Hazard to Others
  
Posts: 117
Registered: 11-5-2005
Member Is Offline
Mood: annoyed
|
|
The diagram looks fine. I don`t know the reaction works esay with HCL. Rather than with a other solution or the way from a existing nitramide to a
nitramin ?
It have likely better attributes (density ad VOD) as TNGU and DNGU.
I have attached a diagram to get a THD (Tetrahedran) nitramin or nitramide derivat maybe with the same way, but diagram not uploadable  ? ?
The formulas here.
(NNO2)3CNO2
(NHNO2)3CNHNO2
[Edited on 26-12-2005 by Madandcrazy]
|
|
|
Mason_Grand_ANNdrews
Hazard to Self
 
Posts: 63
Registered: 19-1-2006
Location: New Berlin City !!
Member Is Offline
Mood: crabby
|
|
| Quote: |
High-tech laboratory complex is needed to experiment with cyclic nitramines.
|
I belive N2O5 is hard to get, when so ever. You don`t can nitrate higher when the N2O5/HNO3 mix is not available.
Is N2O5 available by homemade, dehydration of HNO3, ammonium nitrate etc. ?
[Edited on 14-6-2006 by Mason_Grand_ANNdrews]
|
|
|
mantis
Harmless

Posts: 38
Registered: 17-7-2005
Member Is Offline
Mood: No Mood
|
|
N2O5 is very instable, afaik you can not store it in the pure form.
P2O5+HNO3 yields N2O5 and HPO3.
|
|
|
Mason_Grand_ANNdrews
Hazard to Self
 
Posts: 63
Registered: 19-1-2006
Location: New Berlin City !!
Member Is Offline
Mood: crabby
|
|
N2O5 is stable at -10 gegree celsius and very hycroscopic.
Your knowledge are correct, N2O5 was easiest avialable by HNO3 and P2O5 ?
I`ve found a articel, N2O5 was manufactured per distillation.
Fuming white HNO3  is destilled with ozon gas to a beaker with freshly prepared
P4O10 which was cooled to -10 gegree celsius and N2O5 should formed in a oxidation process when the ozonylized HNO3 passed to the P4O10. is destilled with ozon gas to a beaker with freshly prepared
P4O10 which was cooled to -10 gegree celsius and N2O5 should formed in a oxidation process when the ozonylized HNO3 passed to the P4O10.
I belive P4O10 is available as a drying agent for the industrie and is formed by the oxidation of phosphors with a lage excess of oxygen.
P4 + 5 O2 -> P4O10
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I do not think "HANA" could be made. The chemistry of formic acid, for example, is very different from that of formaldehyde. I am not sure the
best way to explain this. Certainly, HANA could not be made by simply using formic acid instead of formaldehyde in the usual RDX process.
N-nitroformamide, NO2-NH-CH=O, might form, but there could not be any further condensation. Amides are very stable and usually relatively unreactive
because the amine group is electron donating to the oxygen atom.
So one might speculate what would result from condensing bromoform with nitramide. First it should be mentioned that iodoform is probably not the best
regent to use, because the condensation will produce hydrogen iodide, which is a strong reducing agent, and could potentially reduce the nitramine
groups. This would not be a problem in aqeous solution, since it is only anhydrous HI that is a very reactive reducing agent. But in the
presence of water, the intermediate products of condensation (between CHBr3 and NH2NO2) are likely to be immediately hydrated, forming
N-nitroformamide. So back to the idea of using bromoform, I still think "HANA" would be unlikely to form.
The initial intermediate product that would form would be
Br-CH=N-NO2
But think about what would immediately happen. The bromine atom would have a tendancy to immediately ionize off, because there is a nitrogen atom also
bonded to the carbon. This could actually be a very complicated reaction, and I would not know exactly which mechanism it would proceed through. But
this intermediate would likely not be chemically stable, and would decompose before it had a chance to condense with another nitramide.
A related question would be what results from the bromination (in the absence of water) of RDX. Could this form bromo-RDX? No, I think this is
unlikely. There would instead be other degredation products.
http://www.sciencemadness.org/talk/viewthread.php?tid=908
Quote: Originally posted by BASF  | I wonder wether it would be possible to brominate RDX...
Mild conditions. The problem is, i know few to nothing about nitramine-chemistry.....
|
Quote: Originally posted by PHILOU Zrealone  | halogenation of RDX might work but as you said the C-N link might not survive the halide.
(-CHX-N(NO2)-)3 where X = F, Cl, Br or I
Those haloRDX are also interesting because they might be precursor for couplage reactions of other explosophoric groups.
Maybe a different way of action must be investigated!
CH3-O-CH=O + NH2-NO2 --> CH3-O-CH=N-NO2
3CH3-O-CH=N-NO2 --> (-CH(OCH3)-N(NO2)-)3
(-CH(OCH3)-N(NO2)-)3 +3 HBr(g) --> (-CH(OH)-N(NO2)-)3 + CH3Br(g) or (-CHBr-N(NO2)-)3 + CH3OH |
chloroform typically reacts with organic amines to form isocyanides. "chloroform heated with an excess of n-butylamine yield the same sub- stance
along with n-butyl isocyanide and unidentified material"
So [-]C Ξ N[+]—NO2 might be expected to form as the intermediate product, which would likely spontaneously polymerise to and degrade.
Trinitro-1,2,3-triazine, for example, has never been isolated.
Note that [-]C Ξ N[+]—NO2 is not "nitromethanenitrile", which has the formula
NΞC—NO2
[Edited on 5-3-2012 by AndersHoveland]
|
|
|