| Pages:
1
2 |
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Solubility of hydrated salts compared to their anhydrates
| Code: | = Sodium sulphate solubility in water
Na₂SO₄ (142.04 g/mol)
47.6 g/L at 0°C
139 g/L at 20°C //2.92x greater than at 0°C
Na₂SO₄·7H₂O (268.15 g/mol)
195 g/L at 0°C
440 g/L at 20°C //2.26x greater than at 0°C
Solubility of the heptahydrate at 0°C is 4.097x greater than the anhydrate.
Solubility of the heptahydrate at 20°C is 3.165x greater than the anhydrate.
Anhydrate gains 1.888x its weight when hydrated.
= Copper sulphate solubility in water
CuSO₄ (159.61 g/mol)
143 g/L at 0°C
205 g/L at 20°C //1.434x greater than at 0°C
CuSO₄·5H₂O (249.69 g/mol)
231 g/L at 0°C
320 g/L at 20°C //1.385x greater than at 0°C
Solubility of the pentahydrate at 0°C is 1.615x greater than the anhydrate.
Solubility of the pentahydrate at 20°C is 1.561x greater than the anhydrate.
Anhydrate gains 1.564x its weight when hydrated.
= Sodium acetate solubility in water
NaCH₃COO (83.03 g/mol)
1190 g/L at 0°C
1233 g/L at 20°C //1.036x greater than at 0°C
NaCH₃COO·3H₂O (137.08 g/mol)
362 g/L at 0°C
464 g/L at 20°C //1.282x greater than at 0°C
Solubility of the trihydrate at 0°C is 3.287x less than the anhydrate.
Solubility of the trihydrate at 20°C is 2.657x less than the anhydrate.
Anhydrate gains 1.651x its weight when hydrated.
Data gathered from wikipedia and the sciencemadness wiki. |
I'm trying to figure out what the relation is between the solubility of hydrated salts compared to their anhydrates, but the numbers aren't making
sense and I'm not seeing any obvious patterns.
There's one other thread on this from 2012 but it's inconclusive.
can someone explain what the deal is here?
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I didn't double check your numbers, but if there is a difference in solubility when comparing the hydrated salt and the not hydrated salt it comes
from the person determining the solubility. Not the fact the salt was hydrated or not.
Salts don't have a memory, once in solution they are the same, just sodium and sulfate in water for example.
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by Tsjerk  | | I didn't double check your numbers, but if there is a difference in solubility when comparing the hydrated salt and the not hydrated salt it comes
from the person determining the solubility. Not the fact the salt was hydrated or not. |
Well.. could you double check my numbers because the sources I'm looking at definitely say that there is a difference in the solubility of the
hydrated and anhydrous salt. Not only that, the temperature curves are different as well.
http://www.sciencemadness.org/smwiki/index.php/Sodium_sulfat...
http://www.sciencemadness.org/smwiki/index.php/Copper(II)_su...
https://en.wikipedia.org/wiki/Sodium_acetate
|
|
|
teodor
National Hazard
   
Posts: 924
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
There could be differences when water is coordinated to the metal center.
But it depends on the method of the solubility measurement.
There are some (quite rare) anhydrous salts that dissolve in water very slowly but in hydrated form their dissolution is fast.
It is close to the behavior of "calcined oxides" toward acids. It could be the case that after some ion concentration the speed of going anhydrous
salt to the solution is really slow.
If one tries to measure solubility in an opposite way, concentrating solution, the difference in numbers could be because at different temperatures
precipitated hydrates have a different composition.
As for Na2SO4, its solubility is really weird and is a subject of many investigations. I have some sources of information in German language about it,
so please let us know if you are interesting in this particular compound.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
An example of what teodor mentions about slowly dissolving anhydrous salt is Fe2(SO4)3. This salt very slowly dissolves in water. The hydrate,
Fe2(SO4)3.12H2O, quickly dissolves. But I would expect that in the end, the anhydrous salt dissolves to the same extent in water as the hydrated salt,
of course taking into account that the hydrated salt also brings its own water with it.
Another interesting example is VOSO4, a pale grey solid, which is insoluble in water, while the blue hydrated salt, VOSO4.xH2O (I do not remember the
value of x anymore) easily dissolves. I once made VOSO4 (can be made by heating VOSO4.xH2O in concentrated H2SO4) and added the H2SO4 with the
anhydrous VOSO4 to water. The water becomes hot, due to the reaction with the H2SO4 and the VOSO4 settles as a grey precipitate. On decanting the
acidic solution of H2SO4 and replacing it with clean distilled water, the VOSO4 remains settled at the bottom as a grey precipitate. One day later it
still was not dissolved. It might be, that in the very long run, the solid does dissolve, but I did not wait that long.
|
|
|
teodor
National Hazard
   
Posts: 924
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
I think we are puzzled here because we think about solubility measurement either during dissolving or during crystallization. In the reality,
solubility is the characteristic of the state of equilibrium. In this state, different forms of hydrates could go out of the solution depending on the
nucleus formation process.
So, there could be several alternatives during crystallization and the solution has a different capacity for keeping different forms of hydrates from
precipitation. (As a rule, the less soluble is the most "stable" hydrated form for the given temperature.)
So, considering this, we really can have different "solubility" for different hydrates, and the example of Na2SO4 * n H2O with its very complex
dependency on temperature shows this.
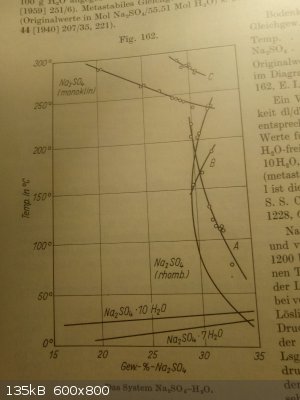
|
|
|
pantone159
National Hazard
   
Posts: 590
Registered: 27-6-2006
Location: Austin, TX, USA
Member Is Offline
Mood: desperate for shade
|
|
The numbers posted above for CuSO4 look sensible: The ratio of molar mass of the hydrate vs anhydrous is 1.564 and the ratio of solubilities at both
temperatures is within 5% of that, so basically both are stated with the same solubility in moles per liter. That seems entirely sensible.
And it sounds like Na2SO4 is a rather peculiar case.
The example in the linked thread, MgCO3, is much more extreme, about a 20-fold difference in solubility. That seems very strange.
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by pantone159  | | The numbers posted above for CuSO4 look sensible: The ratio of molar mass of the hydrate vs anhydrous is 1.564 and the ratio of solubilities at both
temperatures is within 5% of that, so basically both are stated with the same solubility in moles per liter. That seems entirely sensible.
|
Yes, the temperature curves are nearly consistent as well.
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by teodor  | In the reality, solubility is the characteristic of the state of equilibrium. In this state, different forms of hydrates could go out of the solution
depending on the nucleus formation process.
So, there could be several alternatives during crystallization and the solution has a different capacity for keeping different forms of hydrates from
precipitation. (As a rule, the less soluble is the most "stable" hydrated form for the given temperature.) |
I'm not following. Why would the hydration state of the salt before it's dissolved determine the hydration state as it recrystallizes?
As long as the temperature is kept constant, shouldn't the solubility of any hydrate salt be equal to the solubility of the anhydrate salt multiplied
by their molar mass ratio?
|
|
|
teodor
National Hazard
   
Posts: 924
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
Quote: Originally posted by SnailsAttack  |
As long as the temperature is kept constant, shouldn't the solubility of any hydrate salt be equal to the solubility of the anhydrate salt multiplied
by their molar mass ratio?
|
As far as I know, the solubility of different hydrates (expressed as a molar % of anhydrous salt in solution) is really different, so the solution
seeded with crystals of SomeSalt * nH2O with different n deposits different molar amounts of SomeSalt.
I am not an expert on this topic, I just briefly looked at some texts while thinking about your question and these are just my thoughts.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I have been looking at the numbers listed on Wikipedia, stating the solubility of anhydrous Na2SO4 and Na2SO4.7H2O... and they are off a factor two
when looking at the molarity. Now I could be wrong when saying salts don't have a memory or there is a difference I don't understand.
Edit:
According to Wiki a 0.33 M solution of sodium sulfate can be obtained when starting from anhydrous salt, and a 0.73 M solution can be obtained when
starting from the heptahydrate (both 0 degrees).
You can't tell me you get a two fold difference in solubility just by starting with a "different" salt.
[Edited on 23-9-2022 by Tsjerk]
|
|
|
teodor
National Hazard
   
Posts: 924
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
Tsjerk,
I believe your German is fluent.
So it is much easy for you to look through this scan. I believe it contains more reliable data for Na2SO4 solubility than Wikipedia (it is the scan of pages from Gmelin 8 edition, Natrium,
3rd supplement).
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Teodor,
Yes, although I don't like to admit, my German is almost fluent.
What I get from this publication, and I can be wrong, is that they take the water of hydration into account when stating the volume of water needed to
dissolve the salt.
Otherwise I can't explain the difference in solubility.
My understanding in solubility is this: a certain amount in molecules in a certain volume. Let's say 1 mol of a certain salt in 1 liter final volume.
The only way I can explain this would be they take the water of hydration in account (so turning the solubility into mol/gram instead of
mol/(total)milliliter), which could explain the difference. Water is 18 M, a heptahydrate would contribute about a third, but the density increases a
bit, so maybe a factor two... Call me crazy but I can't explain otherwise.
Edit: fuck it, water is 55 molar. I'm out. I don't understand, but I still don't believe a salt becomes more soluble when it was hydrated before.
Edit2: I do have an explanation for the measurements; the behaviour between 0 and 35 degrees is almost exponential, couldn't the two sources be a
factor two off?
[Edited on 23-9-2022 by Tsjerk]
|
|
|
teodor
National Hazard
   
Posts: 924
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
"One part of the 10-hydrated salt requires for solution .. 0.31 parts[ of water] at 33C ... (Gay-Lussac)
At 33C 15.5 atoms of water take up one atom of the anhydrous salt (Brandes & Firnhaber)".
(It is about Na2SO4 from a book published in 1846)
If my calculation is correct, it looks like 10-hydrate requires 8.2 molecules of water per one molecule and anhydrous salt 15.5 to dissolve at 33C.
Well, to take a bigger molecule of 10-hydrate into the solution we have to surround it with a bigger number of water molecules.
And if you ask me, I think there is no "memory" but the solution contains a mix of Na2SO4 with a different number of water molecules in the "inner"
sphere.
[Edited on 23-9-2022 by teodor]
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
So, what I'm reading here is that no one really knows what's going on?
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
You should read more carefully.
Quote: Originally posted by teodor  | "One part of the 10-hydrated salt requires for solution .. 0.31 parts[ of water] at 33C ... (Gay-Lussac)
At 33C 15.5 atoms of water take up one atom of the anhydrous salt (Brandes & Firnhaber)".
(It is about Na2SO4 from a book published in 1846)
If my calculation is correct, it looks like 10-hydrate requires 8.2 molecules of water per one molecule and anhydrous salt 15.5 to dissolve at 33C.
Well, to take a bigger molecule of 10-hydrate into the solution we have to surround it with a bigger number of water molecules.
And if you ask me, I think there is no "memory" but the solution contains a mix of Na2SO4 with a different number of water molecules in the "inner"
sphere.
[Edited on 23-9-2022 by teodor] |
These numbers tell the hydrous and anhydrous dissolve in the same amount of water:
| Quote: | | One part of the 10-hydrated salt requires for solution .. 0.31 parts[ of water] |
So 322.19 grams (1 mol) of Na2SO4.10H2O dissolves in 0.31 parts of water which means 99.88 grams of water --> 5.5 mol
Total amount of water: 15.5 mol
| Quote: | | At 33C 15.5 atoms of water take up one atom of the anhydrous salt (Brandes & Firnhaber)" |
[Edited on 13-10-2022 by Tsjerk]
|
|
|
teodor
National Hazard
   
Posts: 924
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
Quote: Originally posted by Tsjerk  |
So 322.19 grams (1 mol) of Na2SO4.10H2O dissolves in 0.31 parts of water which means 99.88 grams of water --> 5.5 mol
Total amount of water: 15.5 mol
[Edited on 13-10-2022 by Tsjerk] |
Ah, yes, you are right Tsjerk. I miscalculated it.
The question which is open to me is this: "Has the solution only ions Na+ Cl- or also Na(H2O)n+ or undissociated/hydrated salt".
For the elements of the first period, I have the information that Li+ ions exist in solution as Li(H2O)n+ (coordinated) species. And for all other
periods, the metal ions (almost) always coordinate water, so they don't exist as pure metal ions.
For Na I didn't dig into this question. The physics of solution is something I probably will start to understand better some day.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
When ions are in a water solution they will always have a hydration shell, at least I don't know about exceptions. As I understand you can't dissolve
something without a certain interaction with the solvent.
Fun fact: potassium moves through pores (e.g. agar) faster compared to sodium because the hydration shell is smaller.
https://pubs.rsc.org/en/content/articlehtml/2017/sc/c6sc0332...
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Yeah well that math doesn't work for any of the data in my original post. Look:
| Code: |
Sodium sulphate solubility according to the sciencemadness wiki
Na₂SO₄·0H₂O (142.04 g/mol)
139 g/L at 20°C
Na₂SO₄·7H₂O (268.15 g/mol)
440 g/L at 20°C
One mole of Na₂SO₄·0H₂O (142.04 grams) requires 1022 (1000 * 142.04/139) grams of water to dissolve at 20°C.
Total Na₂SO₄: 1 mole
Total water: 56.73 (0 + 1022/18.015) moles
One mole of Na₂SO₄·7H₂O (268.15 grams) requires 609.4 (1000 * 268.15/440) grams of water to dissolve at 20°C.
Total Na₂SO₄: 1 mole
Total water: 40.83 (7 + 609.4/18.015) moles |
The total water is supposed to be the same. It's not. Did I do the calculations right? Why does the data from the sciencemadness wiki show that
anhydrous sodium sulphate require 38.9% more water to be solvated?
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
I think your calculations are correct, but it likely comes down to the variability in solubility data.
Here are a few I found for anhydrous sodium sulphate solubility in water at 20C
SM wiki 139 g/L
Wikipedia solubilities table 195 g/L
US Geological Survey Bulletin 717 194 g/L
Sigma Aldrich data sheet 445 g/L!!!!
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by B(a)P  | I think your calculations are correct, but it likely comes down to the variability in solubility data.
Here are a few I found for anhydrous sodium sulphate solubility in water at 20C
SM wiki 139 g/L
Wikipedia solubilities table 195 g/L
US Geological Survey Bulletin 717 194 g/L
Sigma Aldrich data sheet 445 g/L!!!! |
Jesus christ. We've been at this for hundreds of years and they still haven't gotten the solubility down for even the most basic salts? In water, at
room temperature.
I'll try to pull more solubility data together sometime and run the calculations on them to see if there are any datasets that actually check out. I'm
gonna ignore the weirdies like VOSO₄ that @woelen brought up, at least for now.
|
|
|
teodor
National Hazard
   
Posts: 924
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
I still think there is a difference between "how much water is needed to dissolve" and the solubility as the state of equilibrium of a solid to its
solution.
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by teodor  | | I still think there is a difference between "how much water is needed to dissolve" and the solubility as the state of equilibrium of a solid to its
solution. |
And what properties determine this equilibrium? The mass of the solute and the mass of the solvent. That's just the mass ratio between the water and
the salt. There shouldn't be any discrepancies.
As long as the temperature is kept constant and there's not additional ions in solution there should be complete agreement between the solubility data
on the internet.
There's something very weird going on.
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
| Quote: |
= Sodium acetate solubility based on wikipedia data
NaCH₃COO·0H₂O (82.034 g/mol)
1233 g/L at 20°C
NaCH₃COO·3H₂O (136.08 g/mol)
464 g/L at 20°C
One mole of NaCH₃COO·0H₂O (82.034 grams) requires 66.53 (1000 * 82.034/1233) grams of water to dissolve at 20°C.
Total NaCH₃COO₄: 1 mole
Total water: 3.69 (0 + 66.53/18.015) moles
One mole of NaCH₃COO·3H₂O (136.08 grams) requires 293.28 (1000 * 136.08/464) grams of water to dissolve at 20°C.
Total NaCH₃COO: 1 mole
Total water: 19.28 (3 + 293.28/18.015) moles
Discrepancy of 522%. I'm not sure what to make of this.
= Magnesium sulphate solubility based on wikipedia data
MgSO₄·0H₂O (120.37 g/mol)
351 g/L at 20°C
MgSO₄·7H₂O (246.47 g/mol)
1130 g/L at 20°C
One mole of MgSO₄·0H₂O (120.37 grams) requires 342.9 (1000 * 120.37/351) grams of water to dissolve at 20°C.
Total MgSO₄: 1 mole
Total water: 19.03 (0 + 66.53/18.015) moles
One mole of MgSO₄·7H₂O (246.47 grams) requires 218.1 (1000 * 246.47/1130) grams of water to dissolve at 20°C.
Total MgSO₄: 1 mole
Total water: 19.11 (7 + 293.28/18.015) moles
Discrepancy of 0.42%. The math worked out almost perfectly.
= Copper sulphate solubility based on sciencemadness data
CuSO₄·0H₂O (159.61 g/mol)
205 g/L at 20°C
CuSO₄·5H₂O (249.69 g/mol)
320 g/L at 20°C
One mole of CuSO₄·0H₂O (159.61 grams) requires 778.6 (1000 * 159.61/205) grams of water to dissolve at 20°C.
Total CuSO₄: 1 mole
Total water: 43.22 (0 + 778.6/18.015) moles
One mole of CuSO₄·5H₂O (249.69 grams) requires 780.3 (1000 * 249.69/320) grams of water to dissolve at 20°C.
Total CuSO₄: 1 mole
Total water: 48.31 (5 + 780.3/18.015) moles
Discrepancy of 11.8%... but only if you properly account for the water of hydration of the pentahydrate. If you omit it, the percent error drops to
0.21%, almost as if the data was produced by someone who measured the solubility of the pentahydrate then incorrectly tried to extrapolate the
solubility of the anhydrate by dividing it by the molar mass ratio of the hydrate and anhydrate. They didn't account for the fact that the
pentahydrate's water of hydration contributes to the water of solvation.
|
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
oh my god this is boring
|
|
|
| Pages:
1
2 |