Keras
National Hazard
   
Posts: 929
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Using a(n Ag) mirror RBF to carry out reaction with fused alcali
Folks,
This is very simple: I’m wondering if a glass RBF (or any vessel for that matter) covered in a thin metallic layer, such as the famous Ag mirror,
could be used to perform reactions involving fused alcali such as liquid sodium or potassium hydroxide. It would provide a protective layer against
the alcali, to avoid glass being attacked and dissolved.
Has anyone ever attempted that?
[Edited on 24-10-2022 by Keras]
|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I doubt it would be physically robust enough.
|
|
|
Keras
National Hazard
   
Posts: 929
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
I never attempted making one (I have no silver, though I could buy some of course), but judging by what Nile Red says about it, i.e. that it would
need a spatula or some other tool to be removed, it seems to be pretty solid.
Is there any alternative to it? Like stainless steel RBFs?
|
|
|
pneumatician
Hazard to Others
  
Posts: 412
Registered: 27-5-2013
Location: Magonia
Member Is Offline
Mood: ■■■■■■■■■■ INRI ■■■■■■■■■■ ** Igne Natura Renovatur Integra **
|
|
I think is not a question of thickness, ALWAYS the alkali eat something, your tolerance at this "something" is what matter.
Exist others combos to mirror glass, for example: Pt & Cu, Au & Zn and maybe...
I've a Bronica rotary finder where the mirror layer is corroded and I need to re mirror it, but someone known what type of combo use the Bronica
camera or other brands?? What the combo is better??? what is better in light transmission?? all this is necessary to known before make a decision.
Old SCSI HDD use a glass plate mirroded with Pt... and what more??
|
|
|
Keras
National Hazard
   
Posts: 929
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Quote: Originally posted by pneumatician  | I think is not a question of thickness, ALWAYS the alkali eat something, your tolerance at this "something" is what matter.
Exist others combos to mirror glass, for example: Pt & Cu, Au & Zn and maybe...
|
I'd be happy to know how to make other mirrors. I've tried CuSO₄ reduced by ascorbic acid, it makes very fine metallic Cu powder, but it doesn’t
deposit on the surface of the glass. Pt seems difficult to summon to my needs. Gold mirror?
|
|
|
Twospoons
International Hazard
    
Posts: 1326
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
You should be able to electroplate other metals on top of a silver mirror
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
Fulmen
International Hazard
    
Posts: 1725
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
That's a very good idea. I think nickel is the best choice, at least among the non-noble metals.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Texium
|
Thread Moved
24-10-2022 at 12:03 |
pneumatician
Hazard to Others
  
Posts: 412
Registered: 27-5-2013
Location: Magonia
Member Is Offline
Mood: ■■■■■■■■■■ INRI ■■■■■■■■■■ ** Igne Natura Renovatur Integra **
|
|
to end in the same place, a mirror full of scales.
I think the best is, if can be done, Rh & Pt or one alone, Au... yes if you use Au for photo cameras you see the world yellow :-)
|
|
|
pneumatician
Hazard to Others
  
Posts: 412
Registered: 27-5-2013
Location: Magonia
Member Is Offline
Mood: ■■■■■■■■■■ INRI ■■■■■■■■■■ ** Igne Natura Renovatur Integra **
|
|
Au gilding of glass.
covert the part with a super-saturated borate de soda and applying Au leaf upon it. Afterwards is fixed by burning. Maybe if is for some interior
thing like a photo finder, clean the glass and put Gold leaf.
The ass method is an amalgam with Ga? and heat...
|
|
|
Keras
National Hazard
   
Posts: 929
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
That’s a good idea. It could certainly improve the chemical resistance, but I’m not sure about the ‘physical’ resistance, although I’m
pretty sure the original silver plating is more than OK
|
|
|
Fulmen
International Hazard
    
Posts: 1725
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
I wouldn't put much faith in the mechanical strength of the silver coating. It is extremely thin, and pure silver is pretty soft. Chemically I think
you're right.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Keras
National Hazard
   
Posts: 929
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Quote: Originally posted by Fulmen  | | I wouldn't put much faith in the mechanical strength of the silver coating. It is extremely thin, and pure silver is pretty soft. Chemically I think
you're right. |
Yet, how thick is standard gold plating in jewellery? And the protective oxide layer over an aluminium foil?
|
|
|
Fulmen
International Hazard
    
Posts: 1725
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Only one way to find out I guess.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
wg48temp9
National Hazard
   
Posts: 786
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by Keras  |
I'd be happy to know how to make other mirrors. I've tried CuSO₄ reduced by ascorbic acid, it makes very fine metallic Cu powder, but it doesn’t
deposit on the surface of the glass. Pt seems difficult to summon to my needs. Gold mirror? |
The glass needs to be very clean and for best results sensitized. For example:
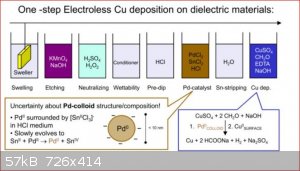
From: https://www.researchgate.net/publication/274009270_Surface_m...
There is lots of info about silvering and electroless copper or nickel on the internet including youtube videos. The are also alternatives sensitizers
to Pd but apparently Pd is the best.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
Twospoons
International Hazard
    
Posts: 1326
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
Electroplating would allow the build up of a thick metal layer, such that the metal to glass adhesion is not really important and the glass merely
provides mechanical support to a metal shell. Thermal expansion might become an issue though. This might be one of those times where soda glass is
better than borosilicate.
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
paulll
Hazard to Others
  
Posts: 112
Registered: 1-5-2018
Member Is Offline
Mood: It's fine. Really.
|
|
Quote: Originally posted by Keras  | Quote: Originally posted by pneumatician  | I think is not a question of thickness, ALWAYS the alkali eat something, your tolerance at this "something" is what matter.
Exist others combos to mirror glass, for example: Pt & Cu, Au & Zn and maybe...
|
I'd be happy to know how to make other mirrors. I've tried CuSO₄ reduced by ascorbic acid, it makes very fine metallic Cu powder, but it doesn’t
deposit on the surface of the glass. Pt seems difficult to summon to my needs. Gold mirror? |
I got some funky copper mirror reducing CuO with H2 in a dropping pipette.
|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I recall reading once that a Cu mirror can be done using hydrazine. I have yet to try it.
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
The difference in expansion coefficient between the metal and glass would be problem, especially since you are talking about molten hydroxide
temperatures. Part of the problem with melting hydroxide in glass, is that the products of the reaction have a different expansion coefficient than
the glass. Upon cooling, the glass cracks.
The metal layer would have to be very, very, thin and have excellent adhesion. It is difficult to make very thin, non-porous metal coatings outside
of a lab. If you want to try a variety of metal coatings, I would suggest looking around for a good vacuum system to play with. We evaporate various
metals (aluminum, chromium, gold, palladium/gold, copper, etc.) under vacuum by placing a small amount of the metal in a tungsten "basket". This is
essentially a heavy tungsten filament. Since tungsten has such a high melting point, it is use to evaporate other materials onto our samples without
introducing contamination. It's conceptually a very simple process.
There are several issues with evaporative coatings, however. First, the glass surface must be chemically clean for the metal layer to adhere. This
means not only does it need to be aggressively degreased, but also needs to be free of adherent water vapor on the glass. The latter can only be
achieved by heating over 100C under vacuum. The instant the glass is exposed to air again, it is "contaminated" with water vapor.
Second, a chromium "adhesion" layer is needed first, in order for things like copper or silver to bond to glass. The chromium cannot be exposed to
air before the next metal is deposited, or the next metal will not stick. This means that both chromium and silver(etc.) sources would need to be
present at the same time without breaking vacuum.
Third, evaporative coatings are usually too porous. It likely wouldn't be a good enough coating for what you're trying to do anyway. One other thing
that I could suggest is painting the glass with a lead oxide enamel. The glass is heated up enough that the litharge fuses to the glass surface.
Next, it is reduced with hydrogen or alcohol vapor. This should give you a thin layer of lead, suitable for electroplating. I have to run now.
Have fun!
|
|
|
Keras
National Hazard
   
Posts: 929
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Thanks for that very detailed input! Very clear and informative. TBH, it was only a hypothetic idea. Given the underlying complexity your process
implies, I think the best way is to work out some sort of metallic container on which a standard glass adapter can be safely attached. That might
still pose a challenge in terms of dilatation, but it’s probably much easier to address than using ultra-deep vacuum to deposit metallic vapours on
an ultra-clean and dry glass.
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Are you planning to stir the reaction?
|
|
|
Keras
National Hazard
   
Posts: 929
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Ideally, yes, and that’s the problem, really.
|
|
|
yobbo II
National Hazard
   
Posts: 764
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
You can plate tantalum onto reaction vessells but that is not your original question.
Something like this.
https://www.sciencedirect.com/science/article/abs/pii/S02634...
Yob
|
|
|
Texium
|
Thread Moved
30-11-2023 at 10:50 |