vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Molybdenum III - Phenanthroline complex (?)
Hello! I had some molybdenum powder, so added conc. hydrochloric acid, some of them dissolved, the solution has green color, then I added
phenanthroline and it turned red like iron(III). so I made more con. solutions and precipitate this complex compound. I know Mo(III) is rare, but as I
found some research, its phenanthroline complex does exist. so this very dark red compound is the result.
in live, it really has a red color, ill take a picture under sunlight tomorrow.
 
 
[Edited on 6-9-2022 by vano]
Attachment: Mo(III).pdf (305kB)
This file has been downloaded 290 times
|
|
|
Bedlasky
International Hazard
    
Posts: 1241
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Mo dissolves very slowly in HCl to form Mo(V). The green complex is [MoOCl5]2- ion. So your phenantroline complex is bound to Mo(V), not Mo(III).
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
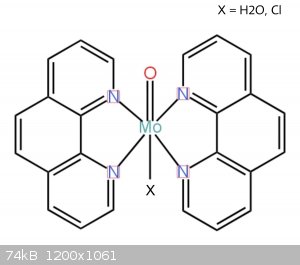
How this structure looks like what you think?
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
new photo:

|
|
|
Bedlasky
International Hazard
    
Posts: 1241
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Molybdenum form usually six-coordinated octahedral complexes. Your complex probably contain Mo=O group with two phenantrolines and one H2O or Cl. But
this is just guess, it could be coordinated on Mo2O4 or Mo2O3 group. If you want to know true composition, you must do some analysis.
[Edited on 8-9-2022 by Bedlasky]
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
thanks, Bedlasky! I made the structure you mentioned. now I can but I'll make some analysis.
[Edited on 18-9-2022 by vano]
|
|
|
vibbzlab
Hazard to Others
  
Posts: 241
Registered: 6-11-2019
Member Is Offline
Mood: Always curious
|
|
I have made a similar complex long back
https://youtu.be/MdiMPaOx98w
It's a phenanthroline, vanadium complex
Amateur chemist. Doctor by profession
Have a small cute home chemistry lab.

Please do check out my lab in YouTube link below
This is my YouTube channel |
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Yes, I have seen it maybe a year ago. interesting complex.
|
|
|