Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Report on making cesium pentaiodobismuthate (lovely red color)
Inspiration to make this came from Poormans Chemist video:
https://odysee.com/@poormanschemist:9/PMC_pentaiodobismuthat...
The reaction was to proceed as below, with a target yield of 10g.
BiCl3 + 2CsCl + 5KI = Cs2(BiI5) + 5KCl
- 2.8g BiCl3 in a small bealer
- Add 1g concentrated HCl and 9g water and stir
- Solution became cloudy, add a few drops HCl until completely clear.
- Dissolve 7.5g KI in 10g water, and add this to the BiCl3 solution. The result was this red solution:

- Dissolve 3g CsCl in 6g water. Add this to the red bismuth iodide solution. A red suspension resulted:

- Stir 10 minutes and gravity filter. Rinse with water in filter twice. A red remainder was recovered with a very pale yellow filtrate. The color with
the eye is very pure red according to my color reference; on the monitor it appears a bit darker:
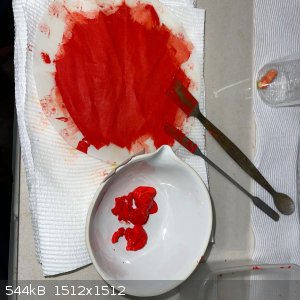
- Dry over NaOH and under vacuum in desiccator for 48 hours. The result was a dry, soft free flowing reasonably heavy powder. Minimal color change
from wet to dry:

- 7.9g bottled, representing a 79% yield. It seemed to stick quite well to the filter paper which probably accounts for some of the loss. Going by
the volume that 7.9g takes up in the vial this product is heavier (denser) than most others I made.

Cleaning the labware with concentrated HCl afterwards showed the product slowly dissolved in HCl into a mostly clear solution.
It will be interesting to see over time how stable the color it; thus whether it slowly decomposes and gives off iodine or not.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4357
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Hmm. I don't have any cesium salts, but I should try making the tetraethylammonium version of this.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
@DraconicAcid Science Essentials have some cesium chloride, I am quite sure Peter will set it in small quantities. Give him a bell if you are
interested.
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Nice complex. I have the same antimony complex Cesium pentaiodoantimonate Cs2(SbI5), I will make a bismuth one.

|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Hi Vano I tried to make the antimonate salt as well but messed up. To get the elemental antimony powder to dissolve quick I added a bit of nitric acid
to the hydrochloric acid and I think some of this nitrate was still present when I added the KI because I ended up with a black solution due to free
iodine. This I managed to wash out the iodine with lots of acetone and I eventually ended up with a product slightly more red than you show above. I
want to repeat this but start with Sb2O3 which should dissolve easier in HCl.
I also tried a cesium lead iodide but I suspect I ended up with only PbI2, because it makes golden rain 
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
nice! yes trioxide is much better. in nitric acid you will get pentaoxide. oxide is also very cheap. i have trichloride reagent, but of course i pefer
to dissolve trioxide in hydrochloric acid.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
My report on making the cesium pentaiodoantimonate. I have a few 100g of antimony powder so prefer to use this and not my small quantity of oxide.
- 6g HCl and 2g 70% HNO3 in beaker (I read somewhere that the presence of nitric acid helps hydrochloric acid to dissolve antimony).
- Add 1.6g fine antimony powder
- After a minute there was a short vigorous reaction with the release of NOx red-brown fumes. After this the reaction slowed down.
- After some hours there was a white suspension. 8g HCl had to be added to get the solution perfectly clear. It was heated to speed up the reaction.
- The next morning there was still a few grains of black material that did not seem to want to dissolve. The solution was filtered. At this point the
filtrate was a pale yellow color, possible due to some dissolved nitrous oxide?
- Dissolved 8.2g KI and 3.3g CsCl in 15g water, result was a clear solution.
- Add the SbCl3 solution slowly to the mix of KI-CsCl. Solution initially turned red, but towards the end of the addition it suddenly turned black. At
the same time purple-red fumes was seen; only for a few seconds and it disappeared before I could smell whether it was iodine vapor or NOx. Probably
unlikely to have been iodine vapor as the solution was at room temp.
- I stopped the stirring and had an orange-red product with black supernatant liquid. I realised it was most likely free iodine.

- Gravity filter. This was the result:

- Wash with 3 lots of acetone in the funnel. This seemed to get rid of most of the iodine, but seemed to also dissolve a bit of the product as the
last drops of wash water was slightly red.

- Transfer the remainder into a beaker and stir for 5 minutes with 100ml acetone:

- Filter. This time there was almost no black color left in the acetone and I was happy that I removed most of the iodine.
- In desiccator over NaOH and under vacuum. After 24 hours it appeared dry, but I left it going for another 24.
- Recovery was 5.6g dry, soft, orange-red flakes that easily powder. This is a 56% yield which I thought was ok after all the acetone washing.

I was surprised to see how the NaOH in the desiccator was discolored. I know NaOH will react with acetone and can form yellow products in the process,
whether this was the only reaction or whether some of the Cs2(SbI5) also carried over I do not know.

EDIT: The color looks very similar to what Vano achieved.
[Edited on 8-7-2022 by Lion850]
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Cesium tetrachloroiodate
I was referencing a Youtube video by Rhodanide:
https://www.youtube.com/watch?v=XtR1yNnxaqI
The procedure calls for potassium iodate, but I did not have any. I did have old discolored sodium iodate. I read that potassium tetrachloroiodate
(the intermediate) was not very stable; I could not find any mention of sodium tetrachloroiodate online but expected that if it did exist to be even
more unstable than the potassium salt. Thus I decided to try a one-pot synthesis.
First batch:
- 4.9g of the old sodium iodate measured out.
- 4.5g cesium chloride in small beaker (a slight excess)
- Add 20g HCl, and stil to dissolve all CsCl (dissolved fast).
- Add NaIO3 in small portions while stirring. There was some bubbling, but not as much as I expected. Hardly any. With each addition there was also a
black color that faded. I assumed this was a sigh of free iodine in my product or maybe NaI.
- The only time I really smelled Cl2 was towards the end of the addition. This was the final solution:

- Add a few grams HCl but the solution remained vary thick. No point to try and filter.
- Scratched all into a evaporating dish:

- Keep dish in desiccator for 48 hours but it hardly dried.
While batch 1 was in the desiccator I progressed batch 2 detailed below. This was much more of a success and I decided to be a bit more bold with
batch 1.
- I dried batch 1 on a steam bath for a few hours. The volume decreased only a bit but it turned more orange. No sign of decomposition.

- Making batch 2 told me that the potassium salt was soluble in water but not the cesium salt. I thus transferred batch 1 into a beaker with 60ml
water and stirred for 20 minutes.
- Vacuum filtered. I got a pale orange yellow filtrate and a bright yellow remainder.

- The remainder was dried under a steel dish in the sun, it quickly dried to a soft bright yellow powder which I am reasonably sure is my cesium
product CsICl4. 2.5g was recovered (initial target was 10g).
- The filtrate was reduced to a wet paste on the steam bath, and then put in a sealed dish of NaOH to dry out slowly. With the eye it looks more
orange than with the phone camera.

Batch 2:
I decided to make fresh KIO3 and have another go.
- 13.8g KOH dissolved in 60ml water in a small beaker suspended in boiling water.
- 30g I2 was measured out and added in small portions, stirring with a glass rod and waiting for the solution to clear in-between additions.
- At 24g the solution remained black. I then added a gram more KOH, then more iodine, and continued so until I had added all 30g I2 and the solution
was a pale olive color.
- While ppt of KIO3 was already forming. The beaker was transferred to a container with cold water. Once cool, the contents filtered and the
remainder, being the KIO3, used wet.
- 15.5g HCl placed in a beaker and the wet KIO3 added in small portions. This time there was a lot of bubbling and pale green color above the liquid,
I am confident that was Cl2 but then realized my cold must have affected my sense of smell more than I realised as I could barely smell the chlorine!

- I added more HCl to try and get all the KICl4 to dissolve, but it would not. I then added 10ml water and it immediately dissolved to a orange-yellow
solution.

- 4.3g CsCl was dissolved in 10g water and then slowly added to the KICl4 while stirring. I thick yellow suspension formed.

- After 15 minutes stirring it was filtered and washed with water in the filter. This is the wet remainder:

- The yellow remainder was placed in a desiccator for 48 hours and the result was 5g of bright yellow product. Representing a 50% yield, more or less.

So in the end quite a nice result, recovery would have been a lot better if I had purer KIO3. The last step remaining is to recover the KI from the
filtrate after the KIO3 was filtered off, there should be a good 30g or so in solution.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Some time ago I made K2FeCl5 and I thought there could well be a cesium equivalent. And online is indeed mention of cesium pentachloroferrate(III); a
few formulas are mentioned including Cs2[FeCl5(H2O)] and Cs2FeCl5·H2O which to me is the same thing.
The plan was to make stoichiometric solutions of FeCl3.6H2O in concentrated HCl and CsCl in concentrated HCl and then combine then, hoping for a ppt.
I immediately encountered a problem. When I opened my old bottle of FeCl3 it was a rock hard layer covered by a layer of solution. I poured the
saturated solution into a beaker, this was about 5g, and managed to break off another 6g in pieces. But I did not know exactly how much I had. 15g HCl
was added and stirred until all was dissolved.
I measured out 7.5g CsCl in a separate beaker with 15g HCl, this should have put the CsCl in excess. Not all dissolved in the acid and I added 5ml
water which then gave a clear solution.
The CsCl solution was added to the FeCl3 solution with stirring. The result was a carrot orange solution, too thick to stir. I added 15ml HCl and
stirred for 15 minutes. This was the final solution (looked more orange with the eye):

The solution was vacuum filtered and the product rinsed in the funnel with concentrated HCl. Below show the wet remainder and the filtrate. I also
noticed the product is slightly soluble in HCl.
 
The wet product was left on the bench overnight but the weight stayed exactly the same. It was then dried on the steam bath. The weight was checked
every 90 minutes and after some 5 hours the weight stopped decreasing and i had a dry, brittle product. The orange in the color became slightly
stronger during the drying.

15.5g was bottled. This would indeed indicate (ignoring losses) that the CsCl was in excess.

Cleaning the implements showed the product to be very soluble in water, giving a faintly yellow-brown solution. Cleaning the implements was easy!
Not sure which cesium compound I will try next, thinking of cesium cobalt nitrite (the potassium salt is yellow) and cesium dichromate.
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Hi
Draconic Acid, did you even get round to making the tetraethylammonium salt?
If you don't, I might try it this week.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4357
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Not yet.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Quote: Originally posted by Lion850  | (Cs2BiI5)
It will be interesting to see over time how stable the color it; thus whether it slowly decomposes and gives off iodine or not.
|
How does the sample keep so far? Any loss of iodine or change of colour?
|
|
|
woelen
Super Administrator
        
Posts: 8030
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I expect it to be quite stable. I have some BiI3 and BiOI, and both can be kept around indefinitely. The complex, described here, also has Bi in
oxidation state +3, and if no air is allowed in, then I see no way for the iodine to split off, because that would require the Bi to go to oxidation
state +2 or +1 and that is definitely not going to happen. If air can get in, then things are different. The oxygen could replace iodine.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Hi Bezaleel all the compounds above have kept the same color at this point.
Hi Woelen - I also tried the cesium copper chloride complex after seeing it on your website.
The first time I tried in water with stoichiometric amounts for Cu2CuCl4, the dry product is brown-orange after drying in a desiccator. I then did it
again in an excess of HCl, the wet product looked like you show but it dried on a steam bath to a red-brown color (with no sign of decomposition). I
never saw any yellow.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Cesium dichromate
Cesium dichromate has a relatively low solubility compared to sodium dichromate and a double displacement between sodium dichromate and cesium
chloride was performed (each dissolved in minimal water).
- 6g Na2Cr2O7 dissolved in 10g water. This gave a clear red solution.
- 9g CsCl dissolved in 10g water. This gave a clear colorless solution. This should have put the CsCl in slight access.
- Add cesium chloride to sodium dichromate while stirring.
- An yellow suspension formed, too thick to stir. Add 10g water and stir for 30 minutes. During the stirring the color quickly changed to light
orange.
- Vacuum filter. Both remainder and filtrate was the same light orange color.

- Wash once in funnel with water (which would have caused some loss of product but hopefully increased purity) and leave on bench to dry.
- After 2 days the product appeared completely dry. The result was 8.2g of soft light/bright orange product, thus an efficiency of some 75%. I don't
know if some chromate also formed, being that the initial suspension was yellow.

- More crystals are also settling out of the filtrate.

This concludes my cesium experiments for now as I want to keep the remaining CsCl in case something special comes up. Below is the family photo, with
the white CsCl being the starting material from the supplier. So far none of the salts shows any color change.

|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Thanks for sharing Lion850, nice work as always.
Not that there is any doubt around what you have created, but I have a small sample of cesium dichromate and it looks just like what you have
prepared.
I also have a sample of cesium formate that was given to me, which is used as a drilling fluid additive. I still haven't decided what to do with it,
though I will definitely consult this page before I decide.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Hi B(a)P thanks for the feedback about the cesium dichromate color. And I then found photos online which shows similar color. What I found interesting
is that the sodium dichromate is a very red salt, as opposed to the orange of ammonium, potassium, and cesium.
I read about cesium formate being used for well control as it makes a very heavy solution. Interesting stuff.
I am keeping an eye open for a cesium salt that is green, blue, or purple 
|
|
|
artemov
Hazard to Others
  
Posts: 181
Registered: 22-8-2018
Member Is Offline
|
|
Sorry to intrude, but can someone help answer my question on making BiCl3(aq) from Bi2O3?
https://www.sciencemadness.org/whisper/viewthread.php?tid=25...
I am interested in making a red salt 
|
|
|