| Pages:
1
2 |
Kobold vor NH4
Hazard to Self
 
Posts: 56
Registered: 27-8-2019
Location: The sewer underneath your house
Member Is Offline
|
|
My first little incident
I thought I would make a separate thread for this.
My first accident in the lab that was serious occurred on the 21st of October 2021 at 2pm (Just noticed the day lines up with the year, dats cool)
I was doing my annual attempt at making phosphorus. I don't try it very often, and I usually fail. I was wanting to make a small amount for my element
collection. If I did this again I would have gone a different route.
This was my idea; Mg3(PO4)2 + 5Mg = 8MgO + 2P
After I had planned out the reaction (not far enough) and all the correct ratios, I started work on it. My lab coat had gone all moldy due to all the
rain, so I didn't wear it, whether or not it would have saved me or made it worse, I don't know.
The total weight of all the reagents was under 10 grams
First I heated some NH4H2PO4 on a hot plate until it did not give off any more ammonia gas, I then very slowly added some Mg powder (I should have
done this part with Mg(OH2)) each time I added it, it would give of H2 and nearly bubble out of the container. After I had added nearly all of the Mg
powder nothing much happened. I was just about to switch it off and call it failed but I saw that something was coming out (probably water vapour), so
I turned up the heat. I had the flask hooked up to a bubbler, I placed it in such a way that I had to reach over the hotplate to move it around and
such, so I reached over with my right hand, one touch of the hose was all it took. BANG!! A massive pure white fireball engulfed my right arm, I
staggered back, first thing I thought was "get to water now!" so I ran without even looking at my arm right towards the tap, and just stood there with
the water running over my arm, looking at the amount of skin that was blasted off of my under fore-arm and lower wrist. The pain did not kick in until
I left the tap to get some aspirin in the hopes that it might ease some of the pain. The pain was intense when I left that tap, I got back to it as
fast as I could, I spent about 15 mins under that tap, no one was in the house at the time, so I tried to call them with our land-line phone, as I do
not have a mobile, I had to go back and forth between the tap and the phone every couple of words I said, the pain was intense. They where already on
the way back, so it didn't take long. Then they took me to the hospital right away. The last time I had been at that hospital for something was when I
was born. So I went in to the ER with my father, I had my hand in a bucket with ice in it, I wonder what some of the other people thought when they
saw that. After about 10 or more mins of waiting in the ER waiting room, they brought me in and got me to flush my arm with water, for another 20
mins, after that they brought me outside a room where they would treat my arm, there was someone being treated in there for some back problems, and
their medic was not supposed to be using that room so that took 5 or 10 mins, meanwhile my arms drying out and getting more painful, but they got me
in and wrapped it in some cling wrap, waited awhile for them to give me a tetnis shot, and some painkillers, then took off the cling wrap, smeared
some fancy Vaseline on my arm, then they wrapped it some antibacteria mesh followed by lots of wadding. Then they let me out and told me to come back
the next day. I had to go back many times to get the arm bandages replaced and my arm cleaned, lots of waiting. At one point they were considering
having to graft a small patch (where the mouth of the flask was) it was charred, but it healed nicely. I consider myself VERY lucky that it was not
worse. It has now made a full recovery, it is still discolored, but that will change after it tans back to the tone of the skin near it. Also the
nerves are only slightly damaged, it is only slightly numb when I touch it. I will post some photos tomorrow.
[Edited on 29-1-2022 by Kobold vor NH4]
|
|
|
metalresearcher
National Hazard
   
Posts: 758
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
Looks like an hydrogen / air explosion. The NH4H2PO4 decomposes upon heating, leaving H3PO4 and NH3 behind. Adding Mg powder to hot H3PO4 releases H2
gas which probably caused the explosion.
Such experiments should be done in a fumehood and the user should wear proper (e.g. leather welding-) gloves. The window of the fumehood acts as a
face shield. When no fumehood, perform it outdoors and wear a face shield.
There was probably no P4 involved, as the reaction he describes requires much higher temperatures.
And experimenting with distilling P4 are dangerous, no risk should be taken that P4 touches your skin, which leaves nasty burns. I did it once in my
fumehood using a stainless steel retort and when I thought it was finished and I removed the retort tube from the water bath, there dripped some
'water' from the retort tube. When it dripped on the fumehood floor, it catched bright yellow flames with white smoke, it was just liquid P4. Luckily
my fumehood is lined with fireproof cell concrete sheets. So I could perform it safely.
So stay safe !
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Glad to hear you are ok. A lab coat is designed to take a bit of heat. It likely would have taken the brunt of the heart and you would have been less
burnt.
|
|
|
Kobold vor NH4
Hazard to Self
 
Posts: 56
Registered: 27-8-2019
Location: The sewer underneath your house
Member Is Offline
|
|
My thoughts on the explosion would have been more around the Mg suddenly igniting, maybe from the air, but the amount of material that was ejected
suggests it was a rapid redox in the mixture. It wouldn't have been just H2, as it was no longer bubbling and the color of the fireball was a solid
bright white. I was cautious of the H2 coming off, but for some reason I did not think about the Mg igniting. A lapse of judgment I guess. I should
have taken more precautions, such as wearing long sleeve leather gloves. I do not have a fumehood, but the shed I was in is extremely well ventilated,
It has two massive openings on both sides for cars and no doors, just square holes where a door should be. I think the lab coat would have melted into
my skin instead of just taking the heat. My arm was right above the opening. The flask was a 50ml schott duran erlenmeyer flask. I'm glad it didn't
break. It has a crack through it now, and its all black. I plan on hanging it up on the lab wall as a reminder, instead of chucking it out.
I can't find the photo of it before I went to the hosptial, But I do have one thats about 4 days after the incident, I had to go back in every 2 of
days to get it cleaned and re-dressed for the next two weeks. The pain subsided after about a week. The burn extends to the other side of the arm too.
I'm the kind of guy that can smile or laugh in most situations, as evidenced by the grin. 
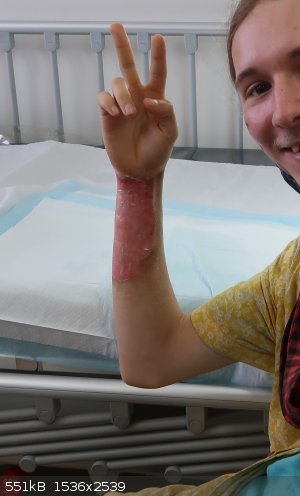
[Edited on 30-1-2022 by Kobold vor NH4]
|
|
|
metalresearcher
National Hazard
   
Posts: 758
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
Probably a combination of H2 and Mg, because Mg alone does not explode or burn violently, unless you have pure oxygen. The H2 explosion ignited the Mg
probably. Moreover, Mg metal in a (hot) aqueous solution does not auto-ignite in air.
Anyway, good that your arm is restored.
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
I think you've just invented a new type of flash powder - that is phosphate-Mg flash powder.
It is known not only nitrates perchlorates etc. can form explosive compositions with active metals like Mg,Al, but also things like BaSO4 (look
youtube impressive bangs), CaSO4 plaster and many other elements in thier higher oxidation states. It seems phosphate is no exception, so for someone
interested I would suggest to try some dry very well ground phosphate+MgAl flash powder for investigation, maybe Ca3(PO4)2, from bones??
|
|
|
MineMan
International Hazard
    
Posts: 1004
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
No one is going to point out that lab coats are cotton, they do not melt but take heat very well. It’s synthetics that are not your friend.
|
|
|
simply RED
Hazard to Others
  
Posts: 209
Registered: 18-8-2005
Location: noitacoL
Member Is Offline
Mood: booM
|
|
Papaya, my thoughts exactly. With a very reactive metal as Mg even carbonates and SiO2 can act as oxidizers, not very effective though... And the
mixture had enough hydrogen to lift the burning Mg and mix with air.
Be careful with Mg, when heating, milling, etc.
When logic and proportion have fallen sloppy dead...
|
|
|
metalresearcher
National Hazard
   
Posts: 758
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
Intereseting. I tried it, but no flash powder, but it did react and probably some phosphorus appeared.
Here my result: http://www.sciencemadness.org/talk/viewthread.php?tid=158331...
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Quote: Originally posted by MineMan  | | No one is going to point out that lab coats are cotton, they do not melt but take heat very well. It’s synthetics that are not your friend.
|
All lab coats should be cotton or wool, but unfortunately that is not the case.
|
|
|
Kobold vor NH4
Hazard to Self
 
Posts: 56
Registered: 27-8-2019
Location: The sewer underneath your house
Member Is Offline
|
|
-MineMan
I should have said "meld into my flesh", not melt. From what I hear it can happen with cotton clothes, But I think that's more from liquid based fires
on the skin with is covered with liquid. I should think most people will know what lab coats are made of, you should always know what your PPE is made
from.
-papaya
That is what I meant by "rapid redox" the Mg metal taking that last oxygen from the Mg phosphate, while the other oxygen's get taken by the ones
already attached to it. I did find the Mg CaSO4 flash mixture by accident, I was trying to use the plaster as a way to slow the burning of it, however
it went off with quite a bang and flash (no accidents that time, I had proper safety gear on that time though). I looked it up later to see if anyone
had found this out and found a post somewhere on here of with the same mixture, apparently they used it in one of the world wars. I can't remember
much more detail, it was a while back, I don't think I would be able to find it.
-Metalreasearcher
I should also mention in my experiment, I did not use straight up Mg Phosphate, I decomposed ammonium phosphate as that is what I had ready to use. So
there would have been residual Phosphoric acid leftover, which may also have contributed to the heat output.
-simply RED
I would say that would be what happened, the hydrogen would have been set off by a tiny spark from the mix as I bumped it, and shot the reaction mix
(which was a thick liquid at that point) and rapidly reacted. BOOM!
[Edited on 1-2-2022 by Kobold vor NH4]
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Glad to hear you didn't need a graft or major debridement.
Those both are nasty.
Hope the family isn't too freaked out by this.
I'd hate to hear your experiments are being curtailed because of one accident.
I think most of us have had at least one nasty accident, even some of the professionals who have the best in equipment and training.
If the family seems nervous it might be good to make a show of beefing up your safety measures a bit.
Maybe even look into building a fume hood if your shed has the room.
You may not need a face shield for the work you do, but buying one (pretty cheap really) and wearing it a lot will probably help them with their
anxieties if they have any.
|
|
|
Kobold vor NH4
Hazard to Self
 
Posts: 56
Registered: 27-8-2019
Location: The sewer underneath your house
Member Is Offline
|
|
--SWIM
My family is quite supportive of my hobby, which is good. They are a bit nervous about the explosive stuff , but they know I'm self-correcting in my
activities. I do have a face shield, but at the time it was grimy, like the lab coat, so I didn't wear it, and I usually only wear it when I THINK I
know that the chance of a hazardous splash/fireball is a possible, however things happen in life that tends to temporarily lower my judgement, as had
happened here.
the problem was that I didn't THINK it would have been such a massive explosion, I had a gut feeling that it might do something, but I didn't follow
it for some reason. Now I guess you could say It has "opened my eyes" more, I clean my bench much more now, and I try to not do a procedure without
thinking it all the way through. The last thing I want to happen is to go through it AGAIN, or through something even worse, like losing a limb, or...
THE BIG ONE!
What I need to get Is a special locker or drawer just for PPE, a nice solid, water tight locker. My labs too open to the weather, things just get
moldy or rot so quickly.
|
|
|
Xanax
Hazard to Self
 
Posts: 54
Registered: 28-8-2003
Location: Sweden
Member Is Offline
Mood: Curious
|
|
When I was a kid (around 13-14 years old), I had a lab in the house we lived in. I had three fire-incidents, the first was with magnesiumpowder, which
ignited and burned my hand, and the other two was with red phosphorus, which one was selfignited, well I was 13 and should not play with those
chemicals, but I mixed the red phosphorus with potassium chlorate...
You know, red phosphorus is in the strip on the matchboxes, and the matchheads are partly made of potassium chlorate, so in touch with each other it
will ignite. And i mixed those chemicals and shaked the pot...
But luckily i have no scars of the burns now, but I have scars from nitric acid burns on my arm, that won't disapper.
When I grow up, I moved to an apartment and I get rid of the most chemicals, I can't have white phosphorus (which I also had), sodium metal, mercury
compounds and a bottle of pure bromine in an apartment building. But then I started to collect radioactive material, but it wasn't legal, so they came
and took all my stuff. Then 5 years later they made a search warrent again in my apartment, because they thought that I have started to make poisons
again (they found some ricin and abrin when they was here the first time). They sealed of the whole block to search my apartment in chemical suits.
Then I was evicted from my apartment, but I didn't have something then, so I had to go to the court to stay here, and I won. So of course I don't have
any illegal chemicals anymore now...
I wish I could have a lab somewere... I have recently gone through the high-scool chemistry and I want to continue somewhere, may I can go to the high
school to do experiments? Now I only have some sulfur, potassium nitrate, hydrochloric acid, acetone and some household chemicals. I don't wanna loose
my apartment.
[Edited on 2022-2-24 by Xanax]
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
There's a reason why this is called a death mix
(It will kill you!)
The spirit of adventure was upon me. Having nitric acid and copper, I had only to learn what the words 'act upon' meant. - Ira Remsen
|
|
|
ManyInterests
National Hazard
   
Posts: 930
Registered: 19-5-2019
Member Is Offline
|
|
I'm really glad you survived it and I hope for a speedy recovery. That sounds both very painful and extremely dangerous. I didn't have any burns, but
I did accidentally create chlorine gas when trying to neutralize the leftover liquid from a hydrazine sulfate synthesis... and I am NEVER going to try
to 'neutralize' it again! When I make hydrazine sulfate, the leftover liquid is going straight down the toilet like everyone advised. I was putting in
calcium hypochlorite and sodium hypochlorite and completely forgotten about the large amount of acid that was probably left over in the liquid. I was
creating chlorine gas without knowing it. I suppose if I do it again I will need sodium bicarbonate first, but the acid is much more dilute and will
not harm the pipes or be anything the sewer system or water treatment can't handle. any leftover hydrazine will be eaten for breakfast by the bacteria
in the sewer.
The only other dangers I had beyond gasses was a runaway nitration when making Keto-RDX, and due to a number of issues I was having I had a runaway
that... my god was terrifying! Thankfully I did it on my balcony and I was able to rush back into my apartment and close the door which sealed off any
entry of gasses. The cloud of reddish-yellow was huge and and the mixture boiled away into nothingness very quickly. I had no burns and I didn't
inhale any of the fumes, but lady luck was smiling on me that day. I can't rely on her for my next time. But I will arrange it so that I don't have
to.
| Quote: |
When I grow up, I moved to an apartment and I get rid of the most chemicals, I can't have white phosphorus (which I also had), sodium metal, mercury
compounds and a bottle of pure bromine in an apartment building. But then I started to collect radioactive material, but it wasn't legal, so they came
and took all my stuff. Then 5 years later they made a search warrent again in my apartment, because they thought that I have started to make poisons
again (they found some ricin and abrin when they was here the first time). They sealed of the whole block to search my apartment in chemical suits.
Then I was evicted from my apartment, but I didn't have something then, so I had to go to the court to stay here, and I won. So of course I don't have
any illegal chemicals anymore now...
I wish I could have a lab somewere... I have recently gone through the high-scool chemistry and I want to continue somewhere, may I can go to the high
school to do experiments? Now I only have some sulfur, potassium nitrate, hydrochloric acid, acetone and some household chemicals. I don't wanna loose
my apartment. |
Damn, that is one hell of an experience. I honestly don't want any run ins with the law and I do not wish to endanger anyone. You had synthesized
ricin? That stuff is deadly. Many, many years ago I thought of making a little bit, but I decided against it. I have no use for it and I don't know
how to safely get rid of something that dangerous.
|
|
|
mrhyde
Harmless

Posts: 1
Registered: 30-5-2022
Member Is Offline
|
|
You mention that you thought water vapour was coming off. Mg doesn't react well with liquid water, but reacts vigorously with steam producing H₂ +
MgO and creates a very bright white light.
|
|
|
Kobold vor NH4
Hazard to Self
 
Posts: 56
Registered: 27-8-2019
Location: The sewer underneath your house
Member Is Offline
|
|
Quote: Originally posted by mrhyde  | | You mention that you thought water vapour was coming off. Mg doesn't react well with liquid water, but reacts vigorously with steam producing H₂ +
MgO and creates a very bright white light. |
Just read through and I find I did not mention water vapour. Everything was well dried before I did the experiment.
The liquid that I was adding Mg to was (NH4)3PO4 heated to its melting point (190'c-200'c) until it decomposed to form H3PO4.
But there's still the possibility it might have been steam.
Oh yeah, an update on my arm, it healed completely 4 months ago.
Only close up you see the skin looks slightly different, most of the hairs on the top and side have grown back too, hair doesn't really grow on the
under side. It is essentially just normal skin now, feels a little bit rougher than the skin around it, but other than that it's all good. Maybe it
was not as severe because I was so quick to get it to water, or (most likely) it was the hospital staff.
"I don't need no excuse for being what I am"
-----Frank Zappa
|
|
|
ManyInterests
National Hazard
   
Posts: 930
Registered: 19-5-2019
Member Is Offline
|
|
I need to mention that I had an incident a few days ago with nitric acid. I made a fair amount of nitric acid (almost 300ml) and I was aiming for
fuming nitric acid, and I was distrustful of even vinyl gloves, so I opted to go barehanded.
When measuring the density of my yield I had an accidental spill on the back of my hand. A bit of it. Not that much, fell down (it was no hotter than
15C. Quite cold) and it really burned. I reacted quickly with pouring the carbonate solution all over my hand, and then ran to the faucet and washed
my hand for a few minutes with cold running water. I then poured baking soda all over my hand before letting the cold water run on it for a longer
time.
It's been a few days since that incident and I will attach with the pictures. I am taking one every time before I go to bed to monitor the progress.
What kind of topical treatment do I need for this?
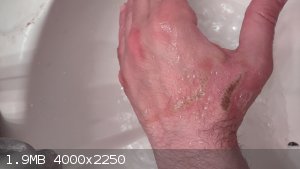   
|
|
|
Keras
National Hazard
   
Posts: 896
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
I’m not sure there’s a topical treatment for this. Nitric acid modifies the structure of keratin and other proteins (coagulation) and that gives
rise to the kind of scars you have. Once you've coagulated a protein, it can’t go back to its previous form. That would be like turning back a
boiled egg into its fresh form.
With time, you should slough it off and it should be replaced by newer, less conspicuous fresh cells.
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Sorry to hear about your injury ManyInterests, I hope that it heals quickly.
|
|
|
ManyInterests
National Hazard
   
Posts: 930
Registered: 19-5-2019
Member Is Offline
|
|
Quote: Originally posted by Keras  | I’m not sure there’s a topical treatment for this. Nitric acid modifies the structure of keratin and other proteins (coagulation) and that gives
rise to the kind of scars you have. Once you've coagulated a protein, it can’t go back to its previous form. That would be like turning back a
boiled egg into its fresh form.
With time, you should slough it off and it should be replaced by newer, less conspicuous fresh cells.
|
I am aware of what nitric acid does to the skin. But in all previous times I just had a faint bit on the fingers that gave the characteristic
yellowing.
But my question is, will it heal mostly fully or will I be left with some ugly looking scars after? Faint scars I don't care much about.
I ended up with (give or take) 75% nitric acid and not fuming nitric acid. I am not sure why as I used mostly pure sulfuric acid (albiet it might not
have been exactly 98%, but it was definitely stronger than drain opener grade and has no impurities) and I dried the potassium nitrate/KNO3 for nearly
12 hours in the oven before adding it to the boiling flask.
I tested it out on both disposable vinyl gloves and latex-free dish washing gloves (because of how thick they are and the added gripping strength).
The nitric acid only stained them (the dish washing glove I used was Nyplex, an Arm & Hammer product). When I have some fuming nitric acid made I
will test it on the dish washing glove to see if it will be chemically resistant to them. The manufacturer states that they are also vinyl and not
nitrile.
|
|
|
Keras
National Hazard
   
Posts: 896
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Fingers and palm have much thicker skin. On the back of your hand the skin is thin and can easily be damaged. I don’t know for the scars, really.
Maybe if you take a fair amount of vitamin C everyday, it could be better (vitamin C helps repair damage and synthesise collagen).
That 75% is weird. Really. Are you certain of your density?
|
|
|
ManyInterests
National Hazard
   
Posts: 930
Registered: 19-5-2019
Member Is Offline
|
|
I am. Took the weight to ml and the temperature. I am quite certain. I will do it again when it comes time to use the acid.
And I mentioned in another thread of how everything seems to be breaking apart. I bought a new 2 liter mantle and boiled some acid with it, only for
it to spit out acid and almost cause a repeat to what happened to my previous mantle. Thankfully I immedately cut power and didn't let the wires burn
out and I first soaked it in bicarbonate water to neutralize the acid and then soak it again in distilled water to try to remove all the bicarbonate.
I used 1.5 liters of distilled water and I am just waiting for my mantle to dry out from the inside.
I don't want my new mantle to burn out. especially when I had it for less than a month!
Edit: my hand is healing quite nicely . The big gash on my thumb is taking a while, but the upper one is starting to peel off. I don't think it'll
leave any real scars. if it does, it'll be very faint and will probably fade away with time. The bigger one will take another 2 weeks I think to
really go away. all yellowness on my skin are gone.
[Edited on 23-7-2022 by ManyInterests]
[Edited on 23-7-2022 by ManyInterests]
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
Scars to the hands are called "The Mark of the Chemist". You are now initiated into the mysteries.
Phlogiston manufacturer/supplier.
For all your phlogiston needs.
|
|
|
| Pages:
1
2 |