| Pages:
1
..
3
4
5
6
7
..
14 |
MineMan
International Hazard
    
Posts: 1014
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Laboratory of Liptakov  | It looks like there has been another breakthrough in the Lithex brisance. The unsuccessful reaction of TACP production from LiClO4 gave a change in
color and consistency after 3 days. The dark blue turned to a light blue suspension. The light blue color is most likely copper hydroxide.
Hydroxy-contaminated LiClO4 was dried. At 180 C. The hydroxide decomposed to CuO. (estimate) A sample of thus contaminated LiClO4 was mixed with
hexamine in the usual ratio of 80:20. The maximum pressure was used when filling the cavity. A density of 1.818 g / cm3 was achieved. For an output
segment weighing 400mg. The brisance at this density is the same as that of highly pressed ETN 400 mg.
Thus, the depth of the crater is 7.5 mm with a crater diameter of 19 mm. Because the tests are shot in lead, an error of at least 10% must be taken
into account.
Anyway, MineMan had truth. If the Lithex can be compressed to high density, its brisance will be identical to ETN. Which has just been confirmed. The
mixture thus prepared has, of course, excellent flame sensitivity. Therefore, it is not necessary to use a different chemical substance for ignition
in the cavity. Lithex with CuO (xy%) works as a monopropelant with incredible brisance.
|
I am confused? Is this hydroxl lithium perchlorate or is this Lithex with a small amount of CuO?
Hexamine is a low density fuel at 1.37g/cc. I advise higher density and easy to obtain fuels for Lithex to achieve the holy 2.0g/cc. The fuels I have
in mind are Melamine at 1.57g/cc and Azodicarbonamide, a widely avilable blowing agent with a density of 1.9g/cc. With the later I predict Lithex @
2.1g/cc easily pressed with brisance between that of RDX and HMX.
|
|
|
MineMan
International Hazard
    
Posts: 1014
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Laboratory of Liptakov  | It looks like there has been another breakthrough in the Lithex brisance. The unsuccessful reaction of TACP production from LiClO4 gave a change in
color and consistency after 3 days. The dark blue turned to a light blue suspension. The light blue color is most likely copper hydroxide.
Hydroxy-contaminated LiClO4 was dried. At 180 C. The hydroxide decomposed to CuO. (estimate) A sample of thus contaminated LiClO4 was mixed with
hexamine in the usual ratio of 80:20. The maximum pressure was used when filling the cavity. A density of 1.818 g / cm3 was achieved. For an output
segment weighing 400mg. The brisance at this density is the same as that of highly pressed ETN 400 mg.
Thus, the depth of the crater is 7.5 mm with a crater diameter of 19 mm. Because the tests are shot in lead, an error of at least 10% must be taken
into account.
Anyway, MineMan had truth. If the Lithex can be compressed to high density, its brisance will be identical to ETN. Which has just been confirmed. The
mixture thus prepared has, of course, excellent flame sensitivity. Therefore, it is not necessary to use a different chemical substance for ignition
in the cavity. Lithex with CuO (xy%) works as a monopropelant with incredible brisance.
|
This makes sense. CuO is an incredible catalyst for perchlorates. It can allow certain flashpowders to DDT. Another little known catalyst is well, I
will say it in private message
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1411
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
I estimate, that is it CuO. But his structure can be different against CuO pure powder, which is simply mixed into white Lithex. During reaction on
TACP and follow within degradation after 3 days, maybe arised better form of CuO. Is it similar as for preparation for example NHN. Temperaure and
entire procedure is important for better or best properties of NHN. Maybe for example 1% of some NH3/4 is bonded in molecule Lithex. I use method of
observation. What it is or not, is not so important. Important is brisance. If is possible preparation make repeatedly with same results. St. Nicholas
may be inside the Lithex molecule if it works. If St. Nicholas create better or biggest hole, let's leave him there...
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
ManyInterests
National Hazard
   
Posts: 942
Registered: 19-5-2019
Member Is Offline
|
|
| Quote: | | Lithium perchlorate can be manufactured by reaction of sodium perchlorate (relatively easy prepare) with lithium chloride which can be cheap and
available. |
Is that like turning Calcium Ammonium Nitrate into Potassium nitrate by adding the CAN in solution with Potassium chloride and letting the potassium
nitrate precipitate? if that is the case, can you give me the ratios please? Or a quick breakdown of the process.
|
|
|
AngelEyes
Hazard to Others
  
Posts: 187
Registered: 24-1-2003
Location: South of England
Member Is Offline
Mood: Better than it used to be.
|
|
Quote: Originally posted by ManyInterests  | | Quote: | | Lithium perchlorate can be manufactured by reaction of sodium perchlorate (relatively easy prepare) with lithium chloride which can be cheap and
available. |
Is that like turning Calcium Ammonium Nitrate into Potassium nitrate by adding the CAN in solution with Potassium chloride and letting the potassium
nitrate precipitate? if that is the case, can you give me the ratios please? Or a quick breakdown of the process.
|
Yes, but I don't think I'd want to do it that way.
Sodium Perchlorate is very soluble, which is good. And Lithium Chloride is pretty soluble too (83.5g / 100ml water @ 20C, according to wikipedia) but
the resulting products are also pretty soluble themselves (Lithium Perchlorate 56g / 100ml @ 20C and Sodium Chloride 35g / 100ml @ 20C). You'd have to
remove the NaCl and then boil the Lithium Perchlorate solution, cool it, filter it, boil some more etc.
Sounds like a PITA to me.
\'Silk and satin, leather and lace...black panties with an Angel\'s face\'
|
|
|
ManyInterests
National Hazard
   
Posts: 942
Registered: 19-5-2019
Member Is Offline
|
|
Quote: Originally posted by AngelEyes  | Quote: Originally posted by ManyInterests  | | Quote: | | Lithium perchlorate can be manufactured by reaction of sodium perchlorate (relatively easy prepare) with lithium chloride which can be cheap and
available. |
Is that like turning Calcium Ammonium Nitrate into Potassium nitrate by adding the CAN in solution with Potassium chloride and letting the potassium
nitrate precipitate? if that is the case, can you give me the ratios please? Or a quick breakdown of the process.
|
Yes, but I don't think I'd want to do it that way.
Sodium Perchlorate is very soluble, which is good. And Lithium Chloride is pretty soluble too (83.5g / 100ml water @ 20C, according to wikipedia) but
the resulting products are also pretty soluble themselves (Lithium Perchlorate 56g / 100ml @ 20C and Sodium Chloride 35g / 100ml @ 20C). You'd have to
remove the NaCl and then boil the Lithium Perchlorate solution, cool it, filter it, boil some more etc.
Sounds like a PITA to me. |
I'm very open to suggestions. Especially since I don't want my lithium perchlorate to be contaminated by sodium chloride. What would be a more
efficient process? Or how would I remove the NaCl from the solution?
I guess that method is good when using it to make potassium nitrate, since the resulting product is quite pure and I've used it to make both the
65-70% azeotrope and the 99.5% fuming nitric acid (I got a specific gravity of 1.51 on my first attempt! It was still a bit red due to nitrogen dixode
contamination, but those can clear up on their own after a while.
|
|
|
underground
National Hazard
   
Posts: 704
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Quote: Originally posted by ManyInterests  |
I'm very open to suggestions. Especially since I don't want my lithium perchlorate to be contaminated by sodium chloride. What would be a more
efficient process? Or how would I remove the NaCl from the solution? |
Direct electrolysis of lithium chloride to perchlorate. Lithium chlorate 1st with a MMO anode and then to perchlorate with a Pt/LD anode. Liclo4 is
much less soluble than Liclo3, so Liclo4 will ppt out of the solution.
[Edited on 28-2-2022 by underground]
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Quote: Originally posted by underground  | [
Direct electrolysis of lithium chloride to perchlorate. Lithium chlorate 1st with a MMO anode and then to perchlorate with a Pt/LD anode. Liclo4 is
much less soluble than Liclo3, so Liclo4 will ppt out of the solution.
[Edited on 28-2-2022 by underground] |
Any reason you wouldn't go from LiCl to LiClO3 with Pt?
|
|
|
ManyInterests
National Hazard
   
Posts: 942
Registered: 19-5-2019
Member Is Offline
|
|
| Quote: | | Direct electrolysis of lithium chloride to perchlorate. Lithium chlorate 1st with a MMO anode and then to perchlorate with a Pt/LD anode. Liclo4 is
much less soluble than Liclo3, so Liclo4 will ppt out of the solution. |
I got an MMO anode and a titanium cathode. So all I need to make a perchlorate is to get a platinum anode? How I do electrolysis is using a DROK
voltage regulator and a 12V laptop power supply. Last time I did electrolysis I managed to get a voltage of 6 and 4.5 or so amps. My yields were...
not good, but I did get get yields nevertheless. It also my first really pure yield of potassium chlorate at the time and I considered it good enough
and I haven't dealt with chlorates since.
I found an online source for lithium chloride, but they only sell for 100 grams a package. I hope that'll be enough for a good long time!
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1411
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Platinum requires relatively precise reaction conditions. Temperature, Ph, voltage, current. Otherwise, it will be destroyed within a few hours. I
recommend the method of thermal decomposition NaClO3 = heat 600C = NaCl + NaClO4.
It's tried, easy and it works.
https://www.youtube.com/watch?v=1Ylt2ZKJlME&t=17s
Also you can try LiCl = electro cell = LiClO3 ....heat.... LiClO4 + LiCl....Unfortunately not tested.
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
...or 500C/20 min. Removing chlorate from the product is easy with K but will be difficult with Na.
BTW a hexamine reference I didn't note before:
https://www.sciencemadness.org/whisper/viewthread.php?tid=25...
Quote: Originally posted by AngelEyes  | | You'd have to remove the NaCl and then boil the Lithium Perchlorate solution, cool it, filter it, boil some more etc. |
I wonder how far from precisely that this was what was going on during the recent incident at BYU (google image: byu dorm room)...or did it involve
sugar, or what exactly...If one was to convert the Na to the Li salt directly, it would be done hot, and the Li would simply be collected on cooling.
Perhaps the recovery of the remainder of the Li (in any form) from the solution at that point would be an issue.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1411
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
It will be easier to prepare perchloric acid and react it with lithium hydroxide. (Li + H2O = LIOH) Until neutral reaction to pH. It's simple, fast
and tried. Pure LiClO4 and water are formed. There are no problems with the reaction. There is also no problem with the production of HClO4 when using
the Adams method. https://www.youtube.com/watch?v=KXpGlgj9_uw&t=70s
It is still easier than counting and testing different solubilities and crystallization conditions and then being frustrated by the tragic yield.
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
Microtek
National Hazard
   
Posts: 872
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
I hate to sound like a broken record, but if you are worried that your NaClO4 is contaminated with chlorate, one solution is to convert it to HClO4 by
reacting with strong HCl (>35%). NaCl is practically insoluble in this and precipitates out. After filtering the NaCl off, you distill the
filtrate to collect the excess hydrochloric acid. By raising the pot temperature to ca. 120-150 C, you ensure that any HClO3 present is thoroughly
destroyed, and all HCl is driven off, while losing almost no HClO4. The product I made this way was found to be 50% w/w, gave no precipitate when
added to dilute AgNO3 (so no chloride present), and did not react with sugar (I didn't have a more sophisticated test for chlorate than this, but I
struggle to imagine ClO3- surviving in a strongly acid, hot environment for prolonged periods).
The only real issues here are access to strong HCl (if the concentration is too low, the solubility of NaCl increases) and whether you can filter such
a corrosive mix. I use a glass frit Büchner funnel which are cheap and widely available.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1411
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
The repetition this method is sure not unnecessary, it is important. It sounds a like simpl method. Simpler than Adams. Because NaClO4 is easer
available and HCl also. Against NH4ClO4 and HNO3 for Adams method. You have some ratios for HCl 35% + NaClO4 (anhydride?) I estimate, that this method
require outdoor conditions during a boiling HCl mixture at 150 C.
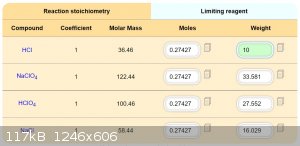
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
Microtek
National Hazard
   
Posts: 872
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
I have attached the paper were I read about this method. In summary, 25-30 ml HCl to 20 grams of NaClO4 (and then several 1 ml portions to wash the
filter cake). Yield is more than 95%. My own results matched those in the paper. And yes, you definitely need ventilation when evaporating the excess
HCl. I collected it by distillation because I didn't want to expose my fume hood pump to large quantities of HCl (and also it can be used for less
demanding applications, such as cleaning glassware, etc.)
Attachment: Mathers_JACS_1910.pdf (377kB)
This file has been downloaded 411 times
[Edited on 2-3-2022 by Microtek]
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1411
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Mathers method seems to be the easiest way to prepare HClO4. HClO4 is, of course, the basic and starting precursor for the research of perchlorates
energetics mixtures. HClO4 is actually responsible for the discovery of a mixture of CuClO4 + hexamine=Cu8. And subsequently for the discovery of
LiClO4 + hexamine = Li8 = Lithex. It is almost certain that whoever has HClO4 can easily come up with another discovery of primary-secondary material.
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
Microtek
National Hazard
   
Posts: 872
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Yes, there is a huge number of perchlorate salts and complexes with nitrogen rich counterions and/or ligands waiting to be discovered. Many of them
will undoubtedly be very powerful, and maybe there will be some that combine high performance and low sensitivity.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
If one is going to use LiOH, they might as well make AP from crude perchlorate and add the hydroxide to a solution of AP and remove the ammonia by
distillation. It would be interesting to substitute with carbonate.
If one is going to use perchloric acid and distillation of HCl, they might as well add LiCl to the acid and get even more HCl, which is presumably
recycled to make more perchloric acid.
|
|
|
Microtek
National Hazard
   
Posts: 872
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
| Quote: |
If one is going to use LiOH, they might as well make AP from crude perchlorate and add the hydroxide to a solution of AP and remove the ammonia by
distillation. |
But then you might not get rid of any chlorate that was in the crude perchlorate. IMO, the increased peace of mind is easily worth the effort.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
The solubility of sodium (per)chlorate is much higher than AP. The 23rd ed. CRC Handbook (1939) sitting here says AP's solubility at 0C is 10.74 g/100
ml water. The only common (per)chlorates less soluble are the K salts and silver chlorate. Perhaps barium chlorate by mole fraction. Ammonium chlorate
is "very soluble".
Quote: Originally posted by Microtek  | | I hate to sound like a broken record, but if you are worried that your NaClO4 is contaminated with chlorate, one solution is to convert it to HClO4 by
reacting with strong HCl (>35%). NaCl is practically insoluble in this and precipitates out. After filtering the NaCl off, you distill the
filtrate to collect the excess hydrochloric acid. By raising the pot temperature to ca. 120-150 C, you ensure that any HClO3 present is thoroughly
destroyed |
I didn't bring up heating HCl with the thermal product for the same reason (plus others) I wouldn't recommend chemical oxidation (in sulfuric acid) of
Na chlorate (but this concern is more avoidable for the K salt, where the process works better in every way, even though technically it should be
chloric acid all the same) to perchlorate: chlorine dioxide and its tendency to explode at concentrations higher than 10%. (2KClO3 + 4HCl = 2KCl + Cl2
+ 2ClO2 + 2H2O)
|
|
|
ManyInterests
National Hazard
   
Posts: 942
Registered: 19-5-2019
Member Is Offline
|
|
Quote: Originally posted by underground  | Quote: Originally posted by ManyInterests  |
I'm very open to suggestions. Especially since I don't want my lithium perchlorate to be contaminated by sodium chloride. What would be a more
efficient process? Or how would I remove the NaCl from the solution? |
Direct electrolysis of lithium chloride to perchlorate. Lithium chlorate 1st with a MMO anode and then to perchlorate with a Pt/LD anode. Liclo4 is
much less soluble than Liclo3, so Liclo4 will ppt out of the solution.
[Edited on 28-2-2022 by underground] |
Quote: Originally posted by B(a)P  | Quote: Originally posted by underground  | [
Direct electrolysis of lithium chloride to perchlorate. Lithium chlorate 1st with a MMO anode and then to perchlorate with a Pt/LD anode. Liclo4 is
much less soluble than Liclo3, so Liclo4 will ppt out of the solution.
[Edited on 28-2-2022 by underground] |
Any reason you wouldn't go from LiCl to LiClO3 with Pt? |
http://www.chlorates.exrockets.com/litpat.html
I've seen this patent that did describe the lithium chloride to perchlorate directly. They did mention Pt anodes for use, but they claimed that even a
graphite anode (which I won't use) would work. I wonder if an MMO anode would work to convert the chloride directly to perchlorate. I have 100 grams
of chloride and given how soluble it is in water, I need to be careful when I try to make it, least I lose everything in one go. I can actually run
that experiment within the next two weeks if an MMO anode will do and not need to shell out a lot of cash for an additional platinum anode (which is a
different size to my cathode).
LL8 will be an interesting challenge to make. I actually forgot that lithex isn't LL8.  But I will be following Liptakov's experiments with lithex very well. If he finds another new energetic that can be used as a primary then
I will be trying it... besides, I gotta use my lithium chloride on something! But I will be following Liptakov's experiments with lithex very well. If he finds another new energetic that can be used as a primary then
I will be trying it... besides, I gotta use my lithium chloride on something!
[Edited on 9-4-2022 by ManyInterests]
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1411
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
For LiCl on LiClO4 you will need PbO2 anode. I recommend all setup from link: https://www.ebay.com/itm/174251053308?hash=item28922c10fc:g:...
Or similar cheaper, necessary find it.
MMO works only on LiCl = LiClO3
For Oxygen 4 in molecule is necessary platinum or PbO2 or Carbon rod.
Carbon rod is almost always decomposed on carbon powder in solution.
Best way as price / power is professional PbO2 anode.
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
ManyInterests
National Hazard
   
Posts: 942
Registered: 19-5-2019
Member Is Offline
|
|
| Quote: |
For LiCl on LiClO4 you will need PbO2 anode. I recommend all setup from link: https://www.ebay.com/itm/174251053308?hash=item28922c10fc:g:...
Or similar cheaper, necessary find it.
MMO works only on LiCl = LiClO3
For Oxygen 4 in molecule is necessary platinum or PbO2 or Carbon rod.
Carbon rod is almost always decomposed on carbon powder in solution.
Best way as price / power is professional PbO2 anode.
|
Those are quite pricey, but I guess I am determined to do it. This will be something I will do a bit later, since I want to try to make ammonium
perchlorate first.
I saw your videos on making sodium perchlorate (which can be used to make ammonium perchlorate by reacting the sodium with ammonium chloride). I saw
that you used a lot of heat to turn the sodium chlorate into sodium perchlorate. Would this process also destroy all the chlorates in the crucible?
All of the work I read on making ammonium perchlorate warn heavily to make sure that no other chlorates are present in the starting perchlorate
otherwise it would convert into the highly unstable and danger ammonium chlorate.
The stuff I also read on lithium perchlorate synthesis is that it can also be produced by reacting lithium hydroxide with ammonium perchlorate, which
will precipitate the lithium perchlorate while converting the hydroxide into ammonium hydroxide. This process seems much simpler since lithium
hydroxide is extremely easy to make.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1411
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Here is method how obtain pretty pure TACP from impurite NaClO4. And then is possible do it decompose on pretty pure NH4ClO4 + CuO.
https://www.youtube.com/watch?v=kUec6kHHxeM&t=3s
Thus TACP preparation in video works a like purification process 2 in 1.
You obtain directly pretty pure TACP. If you need pure NH4ClO4, (for preparation CHP) you can TACP decompose in boiling water on NH4ClO4 + fine
powder (black) insoluble CuO. After evaporate water (separe CuO) you obtain pure ammonium perchlorate.
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Quote: Originally posted by ManyInterests  |
I saw your videos on making sodium perchlorate (which can be used to make ammonium perchlorate by reacting the sodium with ammonium chloride). I saw
that you used a lot of heat to turn the sodium chlorate into sodium perchlorate. Would this process also destroy all the chlorates in the crucible?
All of the work I read on making ammonium perchlorate warn heavily to make sure that no other chlorates are present in the starting perchlorate
otherwise it would convert into the highly unstable and danger ammonium chlorate. |
Most of the chlorate is converted. You can remove the remained with a recrystallisation using acetone.
|
|
|
| Pages:
1
..
3
4
5
6
7
..
14 |