SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Basic iron acetate structure
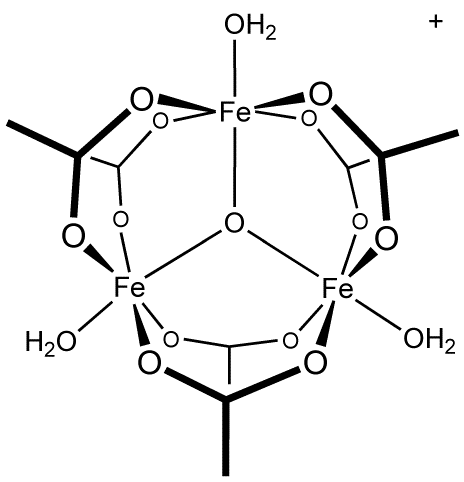
Do you guys know what the deal is with the structure of the basic iron(III) acetate complex? In this picture the water ligands are shown to have a
triple-bonded oxygen, and all the irons have six different bonds. The center oxygen is also triple-bonded.
The complex has a net charge of +1. Does that mean that it usually carries around another acetate anion in solution?
[Fe₃O(OAc)₆(H₂O)₃]⁺
The net formula looks like this. When I produce iron(II) acetate by reaction of vinegar and metallic iron, and then allow that to oxidize to iron(III)
acetate by exposure to air, where is it getting that extra oxygen? Does it hydrolyze some water or pull it out of the air?
|
|
|
CharlieA
National Hazard
   
Posts: 646
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
Do you have a reference to your synthetic procedure and the structure/formula of the product?
I am especially curious that the structure shown does not show (at least to me) acetate ions as ligands. The structure shown shows ether linkages and
no carbonyl C=O bonds.
|
|
|
Texium
Administrator
       
Posts: 4659
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: Preparing to defend myself (academically)
|
|
The water molecules are not covalently bound to the iron, they are just coordinated, which is to say the lone pairs on the oxygen atom (effectively a
concentration of negative charge) are attracted and held in place by the positively charged iron ions, but aren't fully bound. Effectively, the oxygen
in the water is becoming slightly positively charged because the iron is tugging on its lone pair electrons, and the iron is becoming slightly less
positively charged in turn. For practical purposes though, the water is considered to remain a regular old neutral water molecule. It can be bumped
off readily by stronger donor ligands such as amines.
In the case of the central oxygen, it doesn't have three full single bonds. It's technically three "2/3 bonds" for a total bond order of 2 for oxygen,
as usual. Bonding can get really weird when you get into delocalized electrons. We usually talk about whole-number bond orders because it's
impractical to draw fractional bonds, but sometimes electrons can be delocalized (shared across more than one bond), which leads to fractional bonds.
The most common example is benzene, which contains 6 equal length "1.5 bonds" rather than alternating single and double bonds as it is often drawn.
Acetate anion (any carboxylate) is the same way. When a carboxylic acid is deprotonated, the two oxygens become equivalent, with the negative charge
distributed evenly between them, and each C-O bond having a bond order of 1.5. That's why it's able to be a bridging ligand like you see in this
complex and other transition metal carboxylates.
As for the counterion of the complex, it could be whatever was floating around in solution. Could be acetate, but it could just as easily be
hydroxide, or a mixture of both.
@CharlieA: see the attached paper, the crystal structures of these complexes have been known since the '60s.
Attachment: 205694a0.pdf (837kB)
This file has been downloaded 273 times
|
|
|
DraconicAcid
International Hazard
    
Posts: 4403
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I would not say that the water molecules and acetate ions are not covalently bonded. Coordination does involve covalent bonds, even if they are often
a lot weaker than other covalent bonds.
Oxygen can indeed have three bonds, especially in coordination compounds. If you don't consider those to be real bonds, well, there's also things
like oxonium ions (H3O+, (CH3)3O+) or carbon monoxide (which has a carbon-oxygen triple bond).
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by Texium  | | The water molecules are not covalently bound to the iron, they are just coordinated, which is to say the lone pairs on the oxygen atom (effectively a
concentration of negative charge) are attracted and held in place by the positively charged iron ions, but aren't fully bound. ... For practical
purposes though, the water is considered to remain a regular old neutral water molecule. It can be bumped off readily by stronger donor ligands such
as amines. |
ohh that explains the metal aquo complex structures as well
Quote: Originally posted by Texium  | | In the case of the central oxygen, it doesn't have three full single bonds. It's technically three "2/3 bonds" for a total bond order of 2 for oxygen,
as usual. Bonding can get really weird when you get into delocalized electrons. We usually talk about whole-number bond orders because it's
impractical to draw fractional bonds, but sometimes electrons can be delocalized (shared across more than one bond), which leads to fractional bonds.
The most common example is benzene, which contains 6 equal length "1.5 bonds" rather than alternating single and double bonds as it is often drawn.
|
i am somewhat familiar with aromatic bonds. didn't know where else those occurred, that's cool.
Quote: Originally posted by Texium  | | Acetate anion (any carboxylate) is the same way. When a carboxylic acid is deprotonated, the two oxygens become equivalent, with the negative charge
distributed evenly between them, and each C-O bond having a bond order of 1.5. That's why it's able to be a bridging ligand like you see in this
complex and other transition metal carboxylates. |
[img]https://www.researchgate.net/profile/Jeroen-Engelberts/publication/312538062/figure/fig4/AS:669515851526159@1536636426273/The-two-Lewis-resonance
-structures-of-the-acetate-anion-viz-the-conjugated-base-of.png[/img]
i see.
thank you, this'll be helpful as i'm trying to figure out what causes some metal acetates to produce an organic black tar on heating (such as sodium,
calcium, zinc) while others (copper, iron) pyrolyze cleanly. (right now i'm thinking it probably has something to do with whether the metal cation
tries to pull off a few oxygens or an entire carbonate from the acetates on decomposition)
Quote: Originally posted by DraconicAcid  | | Oxygen can indeed have three bonds, especially in coordination compounds. If you don't consider those to be real bonds, well, there's also things like
oxonium ions (H3O+, (CH3)3O+) or carbon monoxide (which has a carbon-oxygen triple bond). |
huh, i guess it does. the internet says that one of those 3 bonds is delocalized like texium was talking about though.
|
|
|
Texium
Administrator
       
Posts: 4659
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: Preparing to defend myself (academically)
|
|
To be clear, DraconicAcid is entirely correct, but covalent bond strength lies on a continuum that isn't limited to single, double, and triple, and
the interaction between a water molecule and a metal ion looks very different from an oxonium ion. So I was just trying to emphasize that most of the
bonds you see in that structure are not "full" single bonds even though it's drawn that way, but it was wrong for me to say that the water molecules
are not covalently bound. They technically are, but it's a much weaker interaction than what you'd normally think of as a covalent bond.
Also side note, aromaticity requires delocalized electrons but not all bonds with delocalized electrons are aromatic... just want to point that out
before you run with any misconceptions. Look up Hückel's Rule for more info about aromaticity.
|
|
|
CharlieA
National Hazard
   
Posts: 646
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Texium  |
@CharlieA: see the attached paper, the crystal structures of these complexes have been known since the '60s.
|
@Texium: thanks for the relevant literature. An old dog can always learn new tricks...or maybe reminded of something that he used to know
(somewhat).
|
|
|
kmno4
International Hazard
    
Posts: 1514
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Freely available (and much newer) literature can be found here:
https://journals.iucr.org/e/issues/2008/08/00/hb2740/
Download the .cif file to enjoy 3-D structure of the iron macrocation ( a free download software "Mercury" is needed).
| Quote: | | The water molecules are not covalently bound to the iron, they are just coordinated, which is to say the lone pairs on the oxygen atom (effectively a
concentration of negative charge) are attracted and held in place by the positively charged iron ions, but aren't fully bound. |
It is oversimplification. It is not easy to remove H2O molecules from hydrated iron(III) chloride/nitrate/acetate/..... etc. There is large difference
between water-iron(III) ion interaction and, for example , water- sodium ion interaction.
The "proof" of covalent oxygen-metal interaction can be reaction: [Fe(H2O)6]3+ + H2O -> [Fe(H2O)5OH]2+ + H3O+
Bonding to transition metal ions in is never so clear and simple.
Слава Україні !
Героям слава !
|
|
|