metalresearcher
National Hazard
   
Posts: 758
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
Crystallizing supercooled Na acetate solution
I found on "The Action Lab" Youtube channel a video about crystallizing sodium acetate (CH3COONa) which can be supercooled.
As I have 'cleaning vinegar' I repeated this experiment. I neutralized some of it with Na2CO3 until CO2 release stopped. Then I evaporated it on an
evaporating dish while still keeping liquid. Then I allowed it to cool in the beaker to let it supercool.
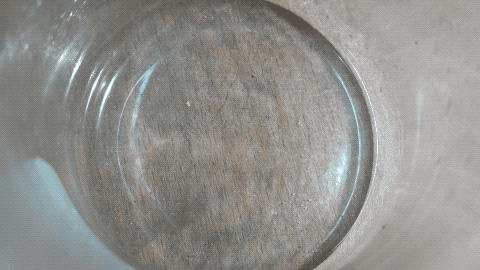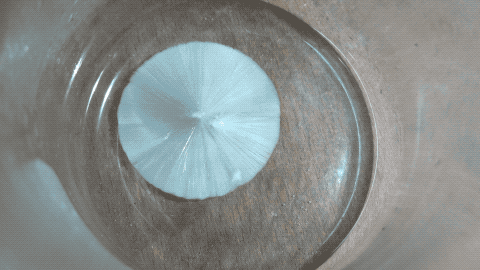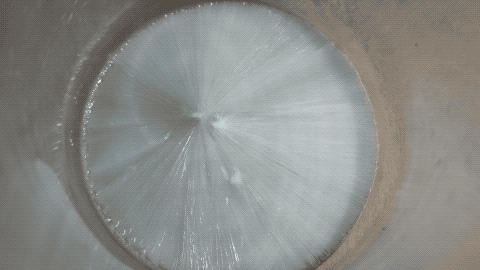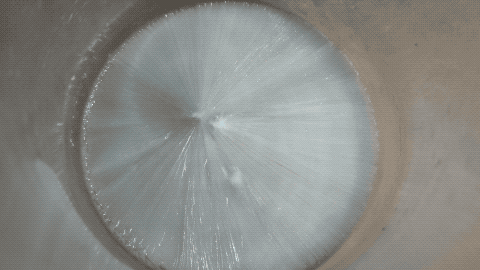
Then I put a tiny grain of Na2CO3 in it and then it crystallized quickly.
Note: this is not a time lapse, but real time ! I was surprised as I thought the YT video was a time lapse while it is actually a real time video.
|
|
|
Bedlasky
International Hazard
    
Posts: 1241
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
This is one of my favorite experiments. Simple, but still quite spectacular. When you take the beaker to your hands, you can feel heat, which is
released during crystalization. Or you can make your own "stalagmite" when you pour supercooled sodium acetate on a surface.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Beautiful!
|
|
|