kilowatt
Hazard to Others
  
Posts: 322
Registered: 11-10-2007
Location: Montana
Member Is Offline
Mood: nitric
|
|
Separating Aluminum Alloy and Getting Calcium Metal Simultaneously
I'm not sure if this reaction would work, since at normal aluminum melt temperatures calcium chloride and other alkali and alkaline earth chlorides
are totally unreactive and are used as fluxes. Maybe at higher temperatures though, this is the idea.
3CaCl2 (l) + 2Al (l) = 2AlCl3 (g) + 3Ca (l)
Silicon from the aluminum alloy would be trapped as CaSi, and at higher temperature the calcium could be distilled off. Then the remaining calcium
silicide could possibly be treated with hydrochloric acid to yield pure silicon.
The mind cannot decide the truth; it can only find the truth.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2828
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
The thermodynamic data for your compounds -- CaCl2, Al, AlCl3, Ca -- are available from public databases, methinks. You should be able to run the
numbers and determine the conditions under which delta-G is negative, if any exist.
http://en.wikipedia.org/wiki/Gibbs_free_energy
|
|
|
walruslover69
Hazard to Others
  
Posts: 236
Registered: 21-12-2017
Member Is Offline
Mood: No Mood
|
|
At a temperature of 1000K the reaction has a delta G of ~840kj/mol so it's safe to say that this reaction isnt taking place
however the same reactions using MgCl2 might be possible if AlCl3 is vacuum distilled off
at 1000k the delta G for the reaction is ~220kj/mol
at 1250K the delta G for the reaction is ~115kj/mol
delta G equals 0 at roughly 1500K
Not sure if this is a viable route.
|
|
|
kilowatt
Hazard to Others
  
Posts: 322
Registered: 11-10-2007
Location: Montana
Member Is Offline
Mood: nitric
|
|
Anhydrous CaCl2 is cheap and easy to buy. But anhydrous MgCl2 would require chlorination of magnesium metal to get. That affects the viability. But I
will give it some thought.
Also calcium metal is a valuable reductant which is otherwise difficult to extract and expensive to buy, where magnesium is not so much.
[Edited on 28-11-2021 by kilowatt]
The mind cannot decide the truth; it can only find the truth.
|
|
|
Vomaturge
Hazard to Others
  
Posts: 286
Registered: 21-1-2018
Member Is Offline
Mood: thermodynamic
|
|
You sometimes see the hexa hydrate for sale as a sidewalk de icer. It's more expensive than the different sodium/magnesium/calcium chloride and
acetate blended de icers and it will be labeled "mag chloride" or magnesium chloride or something similar. It should become anhydrous if slowly heated
to 300C.
Impure MgCl2 solution can probably also be made by mixing strong aqueous solutions of Epsom salt and calcium chloride. Not sure how easy it would be
to clean out the suspended gypsum though.
I now have a YouTube channel. So far just electronics and basic High Voltage experimentation, but I'll hopefully have some chemistry videos soon. |
|
|
kilowatt
Hazard to Others
  
Posts: 322
Registered: 11-10-2007
Location: Montana
Member Is Offline
Mood: nitric
|
|
My understanding is that heating MgCl2 hexahydrate affords MgO and HCl or something similar. Not anhydrous MgCl2. Hence why anhydrous MgCl2 is so hard
to find and expensive if you do find it.
The mind cannot decide the truth; it can only find the truth.
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
300C is listed in Wikipedia as the temperature to make the dihydrate convert to the monohydrate.
The monohydrate gives off HCl under heating according to most sources.
There are patents for drying the chloride by spraying it onto a hot surface which claim the result has a low enough MgO content to be useful in
industry.
But you can't always trust patents.
It is not unheard of for people to patent a process because it looks like it might work and they'd like to have control of the process if somebody
else figures it out and actually makes it work.
The old Selden automobile patent is a famous example of this kind of thinking.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
If the plan worked, Ca wouldn't be made industrially from CaO at 1200C.
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
CaO is cheaper and can be made from limestone in one step. Anhydrous CaCl2 would be more work to get. I don't see how this method is somehow proven
impossible by that simple logic.
My biggest objection to this idea is that calcium isn't too terribly expensive and has a very high boiling point relative to alkali metals, which in
my opinion are more worth producing on one's own. At the kinds of temperatures us amateurs can reliably produce, calcium will be very difficult to
keep in a liquid form once condensed, and building an apparatus is further complicated by the fact you'll almost certainly need to apply vacuum. Even
with a vacuum of 10 Torr, the boiling point of calcium is still over 900C, which is awkward to work with for larger apparatus like the kind you'd
probably need to assemble. And you'd have to distill out AlCl3 beforehand, at whatever temperature that reaction even happens. Not saying it's
impossible, but too involved.
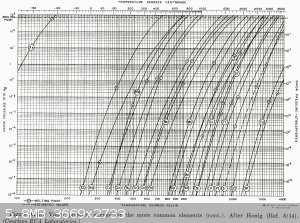
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2171
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
MgCl2.6H2O does decompose but it is not as bad as one would expect.
If too much decomposes you get sorrel cement which is an oxychloride.
heating in a stream of HCl gas will prevent much decomposition and the oxychloride is readily converted to the chloride with HCl.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Quote: Originally posted by Amos  | | CaO is cheaper and can be made from limestone in one step. Anhydrous CaCl2 would be more work to get. I don't see how this method is somehow proven
impossible by that simple logic. |
I did not fail to consider the value of pure AlCl3 over impure alumina, the cost of apparatus for removing and condensing AlCl3 at atmospheric
pressure at let's say the mp of Ca, vs. all that needed for vacuum distillation of Ca at 1200C < 1 mm, or the cost of fueling these operations.
CaCl2 is also made from limestone in one step, with less heat input, and HCl may be someone's waste product.
BTW 50# bags of CaCl2 advertised as pure are $20 at Lowes; if you know of any CaO for less...yes their hydroxide is $11, but it's dolomitic garbage
and chock full of carbonate besides.
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2171
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
If your goal is Ca, may I suggest buying the CaCl2 and electrolyzing it in propylene carbonate.
It is supposedly easy. I plan on making some propylene carbonate and testing this possibility.
|
|
|
kilowatt
Hazard to Others
  
Posts: 322
Registered: 11-10-2007
Location: Montana
Member Is Offline
Mood: nitric
|
|
Making Ca is one goal. Separating 356 aluminum is another goal. My original way to do that was anhydrous chlorination/distillation, getting AlCl3 and
SiCl4. Perhaps generating the anhydrous chlorine off my multi use Downs/FFC Cambridge cell. But that's all a whole lot of work and not scheduled to
happen any time soon.
You've got my interest with the propylene chloride. Where did you get the idea that this would work? Is there a patent or something?
The mind cannot decide the truth; it can only find the truth.
|
|
|