| Pages:
1
..
70
71
72
73
74
..
77 |
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Ketchup?
No….wet chromium picolinate 

|
|
|
CaCl2
Harmless

Posts: 39
Registered: 14-1-2017
Member Is Offline
Mood: No Mood
|
|
Some pictures of my attempts at making YInMn blue.
The first attempt ended up reddish brown, don't know if it was the materials I used or something else. (Made the yttrium oxide from an ebay element
sample, didn't convert the manganse dioxide to manganese(III) oxide first.)
On my second attempt I first got a gray material covered with a thin bright yellow layer. After re-grinding it and heating it more (this time without
it being covered so it got lots of air.), when I took it out of the furnace it looked completely black at first but as it cooled it finally gained a
nice blue color.
I had almost lost hope of ever achieving blue at that point.
  
|
|
|
infrablue
Harmless

Posts: 5
Registered: 25-5-2021
Location: Desert southwest USA.
Member Is Offline
|
|
CuSO4 reacting with KCl in aqueous solution.

|
|
|
infrablue
Harmless

Posts: 5
Registered: 25-5-2021
Location: Desert southwest USA.
Member Is Offline
|
|
Quote: Originally posted by CaCl2  | | On my second attempt I first got a gray material covered with a thin bright yellow layer. After re-grinding it and heating it more (this time without
it being covered so it got lots of air.), when I took it out of the furnace it looked completely black at first but as it cooled it finally gained a
nice blue color. |
It looks great! The color is well saturated.
[Edited on 2021-9-3 by infrablue]
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
The interesting color difference between cobalt and nickel nicotinate tetrahydrates vs the anhydrous salts

|
|
|
Bedlasky
International Hazard
    
Posts: 1241
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
My girlfriend gave me empty bottles which are perfect for making alcohol burners  . Left is H3BO3 in EtOH, right is LiCl in EtOH (in reality it looks more red, on the photo it looks like calcium flame . Left is H3BO3 in EtOH, right is LiCl in EtOH (in reality it looks more red, on the photo it looks like calcium flame  ). ).
Just short question - does H3BO3/EtOH burner create significant amount of ester? I just wondering if I doesn't create some B2O3 aerosol in the room.

|
|
|
itsallgoodjames
Hazard to Others
  
Posts: 276
Registered: 31-8-2020
Location: America Lite
Member Is Offline
|
|
Very colorful picric acid synthesis from salicylic acid



Very colorful picric acid synthesis from salicylic acid. It went from colorless to vibrant red to orange (the pictures aren't in order), then when it
was added to cold water went the expected picric acid yellow. The last image is the recrystallization of it
Nuclear physics is neat. It's a shame it's so regulated...
Now that I think about it, that's probably a good thing. Still annoying though.
|
|
|
Sulaiman
International Hazard
    
Posts: 3721
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
methanol with a nice wick
gives an almost invisible flame,
unlike ethanol which has a yellow tint that makes the desired colours less intense.
its a big difference.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Bedlasky
International Hazard
    
Posts: 1241
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
I also tried methanol later. This time I replaced LiCl with CuCl2. And yes, flame has much better colour.
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Yea, LiCl makes a pleasing red with methanol as seen here briefly in this bottle I used.
https://youtu.be/OUsha3uFPAA
|
|
|
Bedlasky
International Hazard
    
Posts: 1241
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Yeah, I really love that red lithium flame. Everytime I read something about coloured flames or pyrotechnics, everyone always use strontium for red
flames, but lithium make more intense, richer red flame (and also that particular shade of red look nicer than strontium red).
[Edited on 25-10-2021 by Bedlasky]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|

Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Hydrated manganese(II) sulphate

Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
karolus28
Hazard to Self
 
Posts: 51
Registered: 14-4-2019
Location: EU's Brazil
Member Is Offline
Mood: zgrzyt
|
|
potassium chlorate crystal
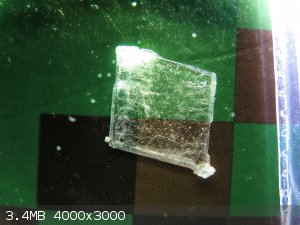
Hi, please read about exif data.
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
IIRC H3BO3 is used as a quick test to distinguish methanol from ethanol, with methanol the flame is green, but it does not color significantly ethanol
you should not rely on such test too much, e.g. if the result is negative (no green flame) you are still not safe to drink such alcohol... I'm writing
this because we all here are of course chemists but some alcohol drinker(s) may visit this forum while looking for the information how to distinguish
these 2 alcohols
I wonder why the esterification proceeds with methanol and not with ethanol, whether it is because of kinetics/polarity/solubility or because common
ethanol usually contains water (most of available ethanol is azeotrope, anhydrous ethanol is very rare). If the reason is the second case then adding
something like 5% water into methanol should prevent green color of the flame while using anhydrous ethanol should color the flame to green.
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Discovered some fluorescent marbles among my mom's collection the last time I visited. The green & white ones are radioactive so I assume it's
uranium. It's the milky part that fluoresces, though, which is different from eg depression glass. No idea what the orange is.
 
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
Admagistr
Hazard to Others
  
Posts: 372
Registered: 4-11-2021
Location: Central Europe
Member Is Offline
Mood: The dreaming alchemist
|
|
Rubies in corundum crucible with MoO3

[Edited on 10-12-2021 by Admagistr]
|
|
|
Admagistr
Hazard to Others
  
Posts: 372
Registered: 4-11-2021
Location: Central Europe
Member Is Offline
Mood: The dreaming alchemist
|
|
Rubies in platinum dish.

|
|
|
arkoma
Redneck Overlord
      
Posts: 1763
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|

After a fairly long battle with cat converter substrate (legitimately sourced!), I'm now the proud possessor of some Palladium Chloride.
Maybe I shouldn't hit the dab before I post as I can't get the image to post' Here's a link. https://photos.app.goo.gl/jr8xHkNmJEpGKyEz5
[Edited on 1-31-2022 by arkoma]
[Edited on 1-31-2022 by arkoma]
[Edited on 1-31-2022 by arkoma]
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Oooohhh. Very nice.
In the lab today, we oxidized fluorene (not fluorine, obviously). One of the students managed to turn it purple when dissolving it in glacial acetic
acid. No idea how that happened.

Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
arkoma
Redneck Overlord
      
Posts: 1763
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
pic

"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Aminoguanidine nickel perchlorate

|
|
|
CharlieA
National Hazard
   
Posts: 646
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DraconicAcid  | Oooohhh. Very nice.
In the lab today, we oxidized fluorene (not fluorine, obviously). One of the students managed to turn it purple when dissolving it in glacial acetic
acid. No idea how that happened.
|
https://en.wikipedia.org/wiki/Fluorene#Acidity. This article may be of some interest. Many different colored compounds are based on polyaromatics.
|
|
|
teodor
National Hazard
   
Posts: 922
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
I am a fan of the "still life" genre in art. I was always amazed how a painter can catch some ordinary mess with proper light angle and color to make
from ordinary household items a piece of art.
It's a pity that painters never use a mess in a chemical laboratory as a subject. It's also a pity that I am not an artist but still, I am a great
master of making a mess. So, once bored with a clean-up I've made my own still-life picture.

|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I'm pretty sure it's not the acidity of fluorene that's responsible here- it may be far more acidic than most hydrocarbons, but not in a protic
solvent like GAA.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
| Pages:
1
..
70
71
72
73
74
..
77 |