| Pages:
1
..
4
5
6 |
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Probably a simpler one than all that.
II. — Action des acides sur l'hexaméthylèneamine et ses composés.
Tous les dérivés de l'hexaméthylèneamine sont décomposés à froid par les acides, l'hexaméthylèneamine elle-même, assez stable à l'état
libre, ainsi que nous l'avons vu précédemment, se décompose très facilement, non seulement en présence d'un excès d'acide, mais également
lorsque l'acide est insuffisant pour former le sel neutre. Suivant la quantité et la concentration de l'acide, il se forme soit de l'ammoniaque soit
une série de bases; nous étudierons d'ailleurs dans un chapitre suivant cette question.
Si l'on chauffe, on obtient de la monométhylamine. Cette réaction a d'ailleurs été signalée, mais au lieu d'être une simple réaction secondaire
sans importance, c'est au contraire une réaction capitale, puisque la transformation est quantitative d'après l'équation:
C6H12N4 + 4HCl + 4H2O = 4(CH3NH2.HCl) + 2 CO2.
Si l'on fait l'opération en tube scellé, à 100-120° pour aller plus rapidement, on constate dans les tubes une forte pression, le gaz qui se
dégage est de l'acide carbonique absolument pur. Il reste dans la solution un peu de chlorhydrate d'ammoniaque, cela tient à ce qu'une portion très
légère de l'aldéhyde formique s'est combinée avec le chlorhydrate de monométhylamine. Cette action se produit avec tous les acides à la
température de l'ébullition. Tous les sels d'hexaméthylèneamine traités de la même façon se comportent naturellement de même.
http://books.google.com/books?id=qqIfAAAAIAAJ&pg=PA394
Whether or not ammonium chloride and formaldehyde is intermediate is not the point, I'm just saying this extra stuff like alcohol or zinc may not
exactly be necessary, even if patents such as DE73812 suggest otherwise.
[Edited on 27-10-2013 by S.C. Wack]
|
|
|
manimal
Hazard to Others
  
Posts: 180
Registered: 15-1-2008
Member Is Offline
Mood: ain't even mad
|
|
Yes, but the book says that the reaction takes place on heating to 100-120C, which is basically the classical reaction between formalin and ammonium
chloride, which hexamine degrades to in hydrochloric acid.
|
|
|
testimento
Hazard to Others
  
Posts: 351
Registered: 10-6-2013
Member Is Offline
Mood: No Mood
|
|
Hexamine is reacted with hydrochloric acid. The reaction is quite simple: one just dumps the hexamine tablets into HCl, lets them sit for few days,
and then refluxes the mixture for 2-3 hours, and after that the water is distilled off, until a mushy white coagulant is acquired. This is mixed with
boiling ethanol, cooled down to room temp, filtered, and mixed with new batch of ethanol and repeated until the ethanol is clear. The ethanols are
mixed and distilled off to leave white mushy residue, which is flushed with chloroform and dried.
Another variation is to use ammonium chloride and formaldehyde, which kicks in when heated up, and then it'll be controlled for a while, and
afterwards water is boiled off.
Aqueous methylamine can be made by mixing the salt with hydroxide and water and brought to boil, and gases lead through cold trap into water, in which
up to 40% solution can be made. The meth boils at -7C, so one can condense it using calcium chlorinated water which freezes at -51C.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Quote: Originally posted by manimal  | | Yes, but the book says that the reaction takes place on heating to 100-120C, which is basically the classical reaction between formalin and ammonium
chloride, which hexamine degrades to in hydrochloric acid. |
What happens in between would be an unimportant detail if their equation would give the maximum yield of methylamine. If there is experimenting to be
done, what they are saying in that article should be where to start. I'm not convinced you simply figure amounts needed to get it to the OS procedure
and that's best.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Some somewhat relevant original literature.
Werner said near the beginning of his isomerization article, "This interesting reaction, which does not appear to have been hitherto recorded"...
...was already recorded by 1880.
https://books.google.com/books?id=BdcsAAAAYAAJ&pg=PA275
There is a big difference here from Werner's 15 minutes @ 275C vs. 2 hrs in a bomb at 300C.
..."Methyl sulphate of ammonium is an unstable salt, decomposable by a moderate amount of heat, while acid sulphate of methylamine is a much more
stable body, hence it becomes probable that if the unstable substance be heated the atoms in the molecules of which it is composed will tend to
arrange themselves in that order which is most stable at the temperature to which they are subjected, and therefore that in this case acid sulphate of
methylamine will be produced. Such, in fact, is the case. A portion of dry methyl sulphate of ammonium was heated by itself in a sealed tube to about
300° C. for about two hours, nearly the whole of the salt being converted into acid sulphate of methylamine.
When the tube, after cooling, was examined, it was found that a very small portion of the salt had undergone a complete decomposition, some carbon
being set free. On opening the tube a small quantity of gas was given off, which was inflammable, and smelt somewhat etherial, with a trace of
sulphurous acid. The salt had been fused, and had solidified to a crystalline mass, and now possessed a strongly acid reaction.
This salt, distilled with caustic potash, and the evolved gas collected in hydrochloric acid, the solution, evaporated to dryness on the waterbath,
gave a hydrochlorate which, on heating with lime, gave off an inflammable gas in abundance, was deliquescent, soluble in alcohol, and when warm
possessed the smell of methylamine hydrochlorate.
Several portions of methyl sulphate of ammonium were heated to different temperatures and for different lengths of time, in order to find out the most
advantageous method of preparing the substance"...
The reaction of sulfamic acid with lower alcohols is naturally quite early and in German.
https://books.google.com/books?id=s-81AQAAMAAJ&pg=PA700
..."Ammonium ethyl sulphate is obtained when finely-powdered amidosulphonic acid is dissolved in absolute alcohol by gently boiling the mixture for
three or four hours. It forms colourless, transparent crystals, is very hygroscopic, dissolves easily in Water, more sparingly in absolute alcohol, is
insoluble in dry ether, and absorbs water from wet ether to form oily drops. It gives up its water over sulphuric acid, and when dry melts at 99°.
The dry salt decomposes above its melting point into acid ammonium sulphate and ethylene, and at 210——220° the latter is evolved in a regular
stream.
Ammonium methyl sulphate, obtained in a similar way, forms hygroscopic leaflets, melts at 135°, and is precipitated from its solution in methyl
alcohol by ether"...
[Edited on 16-1-2015 by S.C. Wack]
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Others have also been more careful and less optimistic than Werner. These authors were also unaware of the earliest work; but optimized similarly.
The Preparation of Methylamirie from Ammonium Methyl Sulphate.
By WILLIAM SMITH DENHAM and LIONEL FREDERICK KNAPP
JCS 117, 236 (1920)
Attachment: CT9201700236.pdf (217kB)
This file has been downloaded 1319 times
|
|
|
Bright Spark
Harmless

Posts: 23
Registered: 1-3-2015
Member Is Offline
Mood: No Mood
|
|
Ok Guys, I am new here, I am trying to get my head around whats legal and whats not
Then I find this thread, doen't this thread break any rules?
Confused
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Here's the FAQ on board topics:
http://www.sciencemadness.org/madscifaq.html#2.1_Board_topic...
| Quote: |
Do not request spoonfeeding of information for clandestine drug manufacture. However, every sort of chemistry is a permissible topic of discussion if
you discuss it like a scientist. If someone is asking about obtaining chemicals used in drug manufacture and using the vocabulary of a SWIM—"someone
who isn't me"—-mer more than a chemist, report it and the thread will be dealt with after a moderator has confirmed your findings. Similarly, if
someone is asking for help with a synthetic "recipe" for a known street drug, report that too. Don't bother to berate the cooks in the thread itself
instead; that doesn't bring the mods any faster. In truth the mods and most members dislike any thread where someone is seeking or using a "recipe"
and doesn't show any deeper interest in chemistry, but this has been mostly a problem with drug synthesis because of the many people who want to
quickly get rich or high.
Certain topics are unwelcome no matter what section they are posted in. The discussion of criminal enterprises or weapons production is inappropriate.
|
If we could not discuss any chemistry or chemical POSSIBLY related to drug production, there might not be too much organic chemistry left to discuss.
On the other hand, starting a thread with "Yo, SWIM givin da big shout out ta Walter White, wutz yer fave shake-n-bake rezipee, dogs?! will not stay
on the organic chemistry forum long.
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Mush
National Hazard
   
Posts: 634
Registered: 27-12-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by kmno4  | I have just evaporated CH3NH3Cl solution [ prepared from absorbing CH3NH2 gas in HClaq; gaseous methylamine was released (by adding NaOH) from brown,
impure product of reduction of CH3NO2 with Fe/HClaq ).
Quickly, cheaply and easily 
On the picture : about 0,6 mole of hydrochloride
[Edited on 26-6-2009 by kmno4] |
Could you share the reaction conditions for us , please?
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
I've done it too, it's easy. I actually use it to test the reducing power of various aluminum alloys I'm researching. Make eutectic gallium/indium
alloy by combining them in a 3:1 mass ratio. Then add 10% of the total mass of that, in tin. (Most lead-free solders are mostly tin, and work well
for this.) Then melt 10x your combined mass of metal, in 1034 or purer aluminum alloy, and mix everything together very well. Allow it to cool
slowly, preferably in a flattened shape. When it gets cool enough to handle, break it up into chunks. These chunks can be dropped into mixtures of
nitromethane containing a protic solvent (lower alcohols, typically), and if your goal is collecting methylamine gas, 30% nitro glow fuel can usually
be used directly. Be aware that suckback WILL happen at some point, and you'll need to condense the methanol and water vapors somehow, or they'll
carry over.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
kmno4
International Hazard
    
Posts: 1514
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Mush  | Quote: Originally posted by kmno4  | I have just evaporated CH3NH3Cl solution [ prepared from absorbing CH3NH2 gas in HClaq; gaseous methylamine was released (by adding NaOH) from brown,
impure product of reduction of CH3NO2 with Fe/HClaq ).
Quickly, cheaply and easily 
On the picture : about 0,6 mole of hydrochloride
[Edited on 26-6-2009 by kmno4] |
Could you share the reaction conditions for us , please? |
Nothing interesting to share, just standard reduction of nitrocompounds with Fe. In this case, because MeNO2 is rather soluble in water, no co-solvent
is needed. Just ~10% HCl + Fe + MeNO2 in some bottle, shaken from time to time until the odour of MeNO2 dissapears (several hours at room temp. but it
strongly depends on frequency and time of shaking). Do not use Fe powder, because it quickly turns to hard mass of Fe/Fe2O3.
Practically, all Fe in converted to Fe2O3 (not chlorides), almost no H2 is evolved, so the bottle can be stoppered and pressure released from time to
time. Impure amine hydrochloride is obtained by evaporation of filtered solution.
Слава Україні !
Героям слава !
|
|
|
Mush
National Hazard
   
Posts: 634
Registered: 27-12-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: |
Nothing interesting to share, just standard reduction of nitrocompounds with Fe. In this case, because MeNO2 is rather soluble in water, no co-solvent
is needed. Just ~10% HCl + Fe + MeNO2 in some bottle, shaken from time to time until the odour of MeNO2 dissapears (several hours at room temp. but it
strongly depends on frequency and time of shaking). Do not use Fe powder, because it quickly turns to hard mass of Fe/Fe2O3.Practically, all Fe in
converted to Fe2O3 (not chlorides), almost no H2 is evolved, so the bottle can be stoppered and pressure released from time to time. Impure amine
hydrochloride is obtained by evaporation of filtered solution. |
This is great, thanx!
Did you use iron sponge (steel wool)?
[Edited on 24-6-2017 by Mush]
|
|
|
kmno4
International Hazard
    
Posts: 1514
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
No, I used iron scraps of all possible kind: corroded and not corroded nails (1-5 cm long), wire (about 1 mm diameter, just was at hand), fragmenst of
cores of old transformers (EI shape), snippets of zinc-coated iron plate.... simply whatever.
I used at least 2-3 molar excess of these scraps, also remember that in first few experiments I used long glass rod to crush this iron mass (from time
to time), but later I used strong shaking only, without of changes in time of reaction.
When the bottle (1 liter volume) is shaking for some longer time (minutes), it becomes warm (~30 C): just reduction accelerates.
Practically, the cost of reductor is negligible.
Слава Україні !
Героям слава !
|
|
|
gatosgr
Hazard to Others
  
Posts: 237
Registered: 7-4-2015
Member Is Offline
Mood: No Mood
|
|
Can't you just react methanol with ammonia to make methylamine?
A possible route but not convenient would be from glycine or trimethylglycine if you decompose them via heat you will get mixtures of tri, di or mono
methylamines then you react with a ruthenium catalyst in the presence of ammonia to make methylamine.
edit: https://www.jstage.jst.go.jp/article/kakoronbunshu/28/1/28_1...
[Edited on 3-2-2018 by gatosgr]
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
When not making P2P, Walter White could have used his thoria tube furnace to make his main object of desire methylamine; things would have gone a lot
better and less dramatically for him if he had.
|
|
|
gatosgr
Hazard to Others
  
Posts: 237
Registered: 7-4-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by S.C. Wack  |
When not making P2P, Walter White could have used his thoria tube furnace to make his main object of desire methylamine; things would have gone a lot
better and less dramatically for him if he had. |
If he was really hardcore he would use this.
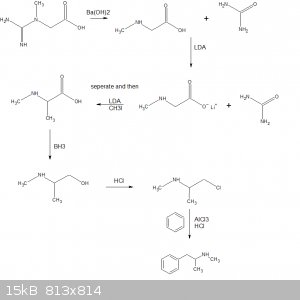
[Edited on 4-2-2018 by gatosgr]
|
|
|
gatosgr
Hazard to Others
  
Posts: 237
Registered: 7-4-2015
Member Is Offline
Mood: No Mood
|
|
I should have added heat to the first reaction.
|
|
|
ketosis
Harmless

Posts: 1
Registered: 1-9-2021
Member Is Offline
|
|
Hi, this may LOOK like my first post. Next topic:
I found a substantial improvement to the sulfamic acid route. This improvement would also be applicable to any route that does not automatically
yield the hydrochloride salt(such as formaldahyde + methanol).
A quick way to explain it:
there's is never any need to gas methylamine into any liquid. ever.
-some claim to be wizards at it, but most seem to agree that it's very very difficult.
I have grown 2 moles of huge ammonium methyl sulfate crystals before. My final MeAm.Cl yield would be like 4 grams. Even with stirring, with a
diffuser. Chilled receiving liquid. etc.
So:
sulfamic acid boils with methanol, after a long reflux, the ester ammonium methyl sulfate is formed.
we know that heating this in a closed container briefly to the upper 200C range will thermally rearrange the contents to a mixture of inorganic
Ammonium.H.SO4 and organic Methylamine.H.SO4, also known as bisulfate.
Turns out that the bisulfate salts, as well as the neutral sulfate salts have the same solubiliy profile as the chloride salts.
Even if it's a black goo.
In fact, not just isopropanol or ethanol, even methanol will hardly dissolve any inorganic ammonium salts, ever. ANd will dissolve organic
alkyl.amine salts easily.
Also chloroform can be ommitted if one has some half-decent crystallization skills, single-solvent, from isopropanol.
I believe this can be applied to the nitomethane route, as well as others.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Quote: Originally posted by madcease  | | What is the best way to go about storing and transfering MeAm HCL from its salt form into a gas canister for storage and 99% anhydrous usage. Ive read
that 50% of base makes the gas but how does one go about filling canister or tank for use later on? |
https://m.youtube.com/watch?v=I_DD2_7QkRk
|
|
|
| Pages:
1
..
4
5
6 |