MineMan
International Hazard
    
Posts: 1012
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Nitroguanidine and sodium hydroxide
A while ago I saw a YouTube video of nitroguanidine 10grams added to 10grams of sodium hydroxide in solution. The temperature rose rapidly and ammonia
fumes evolved. Does anyone know the mechanism and why this basic solution destroys NQ?
|
|
|
fredsci93
Hazard to Self
 
Posts: 84
Registered: 29-12-2017
Member Is Offline
Mood: No Mood
|
|
The reaction starts with cleavage of the nitramine, next the intermediate is either converted to carbamic acid (which subsequently further degrades to
carbon dioxide and ammonia) and ammonia or urea which is more or less stable however at elevated temperatures it also degrades to ammonia and carbon
dioxide. The nitramine from the first reaction is degraded to N2O (laughing gas).
The ammonia is produced in large amounts during the reaction hence the observation of fumes, I drew an electron transfer for the first reaction.
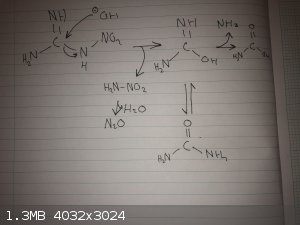
[Edited on 29-8-2021 by fredsci93]
|
|
|
MineMan
International Hazard
    
Posts: 1012
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Thank you so much! I don’t understand because the addition of hydrazine and NaOH produces amminoguanidines. How does a little bit of hydrazine stop
the cleavage of a nitramaine?
|
|
|
fredsci93
Hazard to Self
 
Posts: 84
Registered: 29-12-2017
Member Is Offline
Mood: No Mood
|
|
I believe the main reason hydrazine doesn’t cleave the nitramine and that amino nitroguanidine is not nearly as susceptible to hydrolysis is that
when hydrazine reacts to form amino nitroguanidine it converts the primary nitramine into a far more stable secondary nitramine (I'm pretty sure it's
actually an equilibrium but the secondary form is more favored).
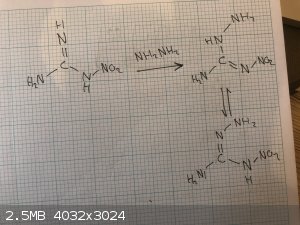
|
|
|
MineMan
International Hazard
    
Posts: 1012
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by fredsci93  | | I believe the main reason hydrazine doesn’t cleave the nitramine and that amino nitroguanidine is not nearly as susceptible to hydrolysis is that
when hydrazine reacts to form amino nitroguanidine it converts the primary nitramine into a far more stable secondary nitramine (I'm pretty sure it's
actually an equilibrium but the secondary form is more favored). |
Ok, but there is NaOH added in addition to the hydrazine. And yet the ANQ forms before the NQ cleaves and is destroyed, why?
|
|
|
MineMan
International Hazard
    
Posts: 1012
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
A third question. Dinitroguanadine exist, tho I cannot find much on it, no performance, no synth, expect that is is very difficult to make. Why would
it be so difficult to add another nitro group to the other NH group?
|
|
|
fredsci93
Hazard to Self
 
Posts: 84
Registered: 29-12-2017
Member Is Offline
Mood: No Mood
|
|
1. the cleavage reaction is I expect much slower than the hydrazine reaction
2. I personally have made Dinitrourea many times and have seen other amateurs on this forum talking about making dinitroguanadine http://www.sciencemadness.org/talk/viewthread.php?tid=17908 there is some literature included in that thread, as for why it's so difficult, it's
for a couple of reasons.
First. Steric effects since the nitro group is nearly as large as the base molecule and it hinders the addition of a second group by repulsion and
destabilizes the dinitro compound.
Second. I assume some resonance effects are playing a part in making the mono nitro product more stable
on the performance, as I understand it is a very powerful explosive which forms many useful salts, the only problem being its synthesis is quite
difficult and requires oleum.
hope this helped
|
|
|