Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Metal picolinates
My interest in chromium picolinate was triggered after reading that it is a bright red chromium compound. I bought food grade zinc picolinate on eBay.
This is a white salt:

Chromium picolinate was made doing a double displacement reaction:
3Zn(C6H4O2N)2 + 2Cr(NO3)3.9H2O = 3Zn(NO3)2 + 2Cr(C6H4NO2)3 + 18H2O
Working out the exact stoichiometry was a bit problematic as I did not know if the zinc picolinate was a hydrate, and my chromium nitrate is also very
old and could have absorbed some moisture by now, although it still seems quite dry.
I also could not find the solubility in water figures for either zinc picolinate or chromium picolinate but I did read somewhere that zinc picolinate
was slightly soluble in cold water but more soluble in hot water, while chromium picolinate was said to be insoluble. I thus did a few trials with
small quantities and then the main run:
- 11g zinc picolinate dissolved in 175ml water, this only dissolved into a completely clear solution at around 95C and after stirring for some 30
minutes (from my trials I knew that 10g zinc picolinate needed 150g near-boiling water to fully dissolve).
- 8.7g chromium nitrate nonahydrate dissolved in 20g water, giving a black solution. This should have put the zinc picolinate in very slight excess.
- Add the chromium nitrate solution to the near-boiling zinc picolinate solution. Nothing seems to happen, you just have a dark solution:

- Switch off the hot plate but leave on the stirring. Let it cool down slowly to room temperature over a few hours. As it cools the solution changes
colour and eventually looks like this:

- I then let the beaker stand overnight, as it was the end of the day. The next was the solution was stirred again to suspend the red ppt that settled
out and then gravity filtered. The reason I don't just simply decant the dark supernatant liquid first is because I found in the trial runs that the
ppt was then difficult to all recover, being fine and sticks to the beaker walls.
- The filtrate was still dark maroon, and the wet remainder was blood red. The remainder was washed in the funnel, and the run-through wash water was
surprisingly clear with just a hint of pink.
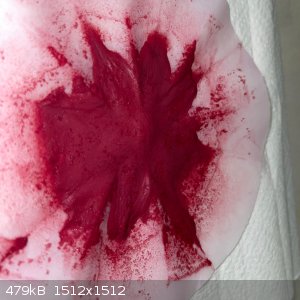
- Dry the remainder in the sun under a steel dish for 6 hours. This gave a very fine, soft, red powder:

- Recovery was 6.2g which is some 65% of the theoretical yield:

To see what was left in the filtrate I steamed it down to 50% of its volume by which time more red-pink suspension was visible. The beaker was left to
cool overnight and this gave a second crop of blood red product, but much less then the main crop. This is now still drying.
The beaker had red stains from the fine powder, and I added concentrated hydrochloric acid to clean it. Hours later the acid had became pink, but not
all the red solid had dissolved.

This intrigued me as it seems this product only dissolved (slowly) in concentrated HCl without reacting with the acid. I poured the pink HCl into a
evaporating dish and heated on a steam bath, and when the solution was very hot and already of gassing copious HCl fumes the colour slowly changed to
almost clear and then green, presumably due to the formation of CrCl3:

- I heated a small sample of the second crop on a steam bath to see if it will decompose. There was no apparent decomposition, but the colour
lightened:
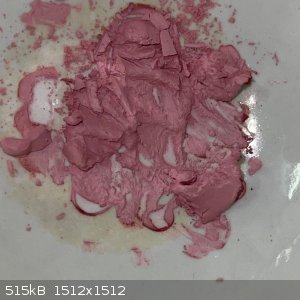
Next I want to try copper.
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
It has very nice colour Lion850
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Thanks for the write-up, Lion, well done 
I'm curious what your final yield will be after you add what you recovered from the filtrate.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Hi Bezaleel the second crop gave 3.5g of dry free flowing soft powder with the same colour as the original crop. This takes total recovery to 9.6g and
thus to just about 100%. And I only steamed down the filtrate to 50% volume once, so there is probably more left in the solution. I did not want to
reduce the volume again as I was worried other components may start to come out. Rather have a bit less chromium picolinate but more pure.
By the way, I found supplier photos of bulk Cr pic online and the colour is just about the same.
So why did I get so much product? I then realised that the filtrate I boiled down also contained the filtrate of the first run which was done using
about 1/3 quantities. And it could also be a hydrate, or maybe not completely dry. As I mentioned above when I put the remainder of the trial run on a
steam bath it turned much lighter in color.
Anyway, it seems a quite straightforward experiment to get a near red compound at good yield.
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
I already wondered whether you could not get a higher yield. But this is good!
I have the same approach - usually throw the last bits away to retain purity. In particular with organic acids, I oftentimes find it really hard to
weigh out a good stoichiometry. I presume that at least one factor of importance is that my organics are not water free and that the crystal water of
my inorganics is neither as accurate a literature would suggest.
Good to see that you colour matches that of a supplier. It's not always easy to even find any sensible photos.
Do you intend to make any other salts from your zinc picolinate?
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Bezaleel I intend to make copper next and then also try cobalt and manganese. I'll probably use the nitrates (Cu, Co, and Mn) as these are quite
soluble and should thus be easy to keep out of the product if in excess. Online I see hydrates are mentioned for most of these picolinates, also a
zinc picolinate trihydrate is mentioned. But I will always assume my zinc picolinate is not a hydrate; I'd rather end us with unreacted nitrate then
unreacted zinc picolinate.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Copper picolinate:
Copper picolinate was to be made as per the below reaction:
Zn(C6H4O2N)2.4H2O + Cu(NO3)2.5H2O = Cu(C6H4O2N)2.2H2O + Zn(NO3)2 + 7 H2O
A zinc pilolinate tetrahydrate is mentioned online, as is a copper picolinate dihydrate. Whether my zinc picolinate is a hydrate or anhydrous I don't
know. I chose the reagent quantities to ensure the copper nitrate is in excess as this is much more soluble than zinc picolinate and should thus be
easier to 'wash' out of the product. Procedure:
- 10g zinc picolinate dissolved in 170g water, as always it only dissolved completely into a clear solution at boil.
- 9.5g copper nitrate dissolved in 30g water, giving a light blue solution.
- Copper pic solution slowly poured into the zinc pic solution near boil and with stirring.
- A dark blue suspension immediately formed. Initially very dark but became a bit lighter, with just a hint of purple which is difficult to capture on
the phone camera:

- The beaker was left to slowly cool on the hotplate while stirring.
- An hour later stirring was stopped and the solution left for 30 minutes to settle. It settled quite fast. The supernatant blue liquid was then
decanted through a filter, 100ml water added and stirred again to remove leftover nitrate, and the lot then filtered. The remainder was then washed
with some 30ml water in the funnel.
- The result was this blue wet remainder:
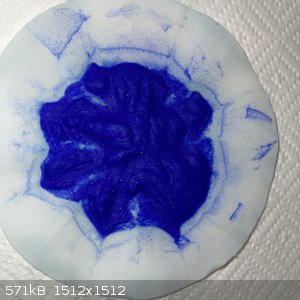
- The wet remainder recovered into an evaporating dish was 18.5g. This was left out overnight but only lost 1g of water.
- The filtrate was to be boiled down to around 70ml to see if anything else crystalizes out. The evaporating dish was placed on top to dry the blue
remainder:

- After 2 hours the weight of the remainder stopped dropping and it appeared dry. It was left on the boiling beaker for another 2 hours but it did not
drop anymore. This is the dry product:

- 7.8g of Safire Blue soft powder (with a hint of purple) was recovered. This is a recovery of some 85% based on the above equation.
- The filtrate was boiled down to around 60-70ml, when hot there was no crystals forming. But once cooled to room temperature lovely blue rod shaped
crystals were observed:
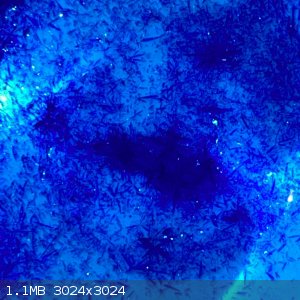
- These were filtered off and is now sitting out on the bench. I suspect this may be excess copper nitrate, if it stays wet or gets wetter it will
support this.
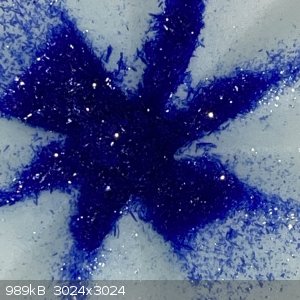
Some comparisons between the chromium and copper picolinates:
- Cr pic appears more soluble than the Cu pic, thus a significate amount of extra Cr pic was recovered after boiling down the filtrate.
- The Cu pic dissolved in HCl at room temp giving a green solution which I think indicates the formation of copper chloride; the Cr pic would only
react with very hot HCl.
- The Cr pic ppt stuck to the beaker and stir bar; the Cu pic washed off easy.
Next will probably be cobalt.
|
|
|
j_sum1
Administrator
       
Posts: 6335
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Those really are some stunning blues there, Lion.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Cobalt:
- Same process as above. A solution containing 9.5g cobalt nitrate hexahydrate was added to a near boiling solution of 10g zinc picolinate.
- No obvious reaction, similar color as the cobalt nitrate solution and no sign of a suspension:

- Leave to cool to room temperature. Still no suspension, which means something DID happen as there seemed to be no more zinc picolinate in solution -
this becomes very insoluble at room temperature and would have fallen out of solution if still present in any significant amount.
- Start to heat again to steam down. At a certain point an orange suspension suddenly appeared:

- But this disappeared again as it heated up.
- Steam down from 170ml to 80ml. At 80ml there was still nothing coming out of solution:

- Leave to cool slowly. The same looking orange suspension precipitated out:

- After standing overnight it was filtered and washed in the funnel. It was then left in the sun for an hour to dry a bit, this resulted in a bronze
coloured damp product:

- It was further dried on the steam bath. As it dried the colour changed to light pink. Final recovery was 8g of light pink powder:

** I cannot find any description or photos of cobalt picolinate online. I will appreciate it if anyone can confirm my product has the right colour **
- Boiled the filtrate down from around 70 to 30ml. Nothing fell out of solution. Let it cool to room temp, still nothing, just the colour darkened as
it became more concentrated. This surprised me as I was expecting to see more of the same pink product fell out of solution.
- Used concentrated HCl to clean the remaining bits of pink powder in the evaporating dish and beakers, it dissolved reasonably easy giving a blue
solution. Which turns reddish when water is added, indicating the behaviour of cobalt chloride.
** Manganese: I tried the double displacement reaction with manganese nitrate but cannot say whether anything happened. Upon cooling a white salt
precipitates out, no hint of pink at all. Whether this is manganese picolinate or just the unreacted zinc picolinate I can't say. The "product" is
pure white and the solution clear.
I am now doing Iron, starting from iron (iii) nitrate.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Iron:
- 10g of zinc picolinate dissolved in 170g water very near boil
- 9.5g iron (iii) nitrate dissolved in 30ml water, giving this brown-orange solution:

- Iron nitrate solution poured into the hot zinc picolinate solution under stirring. The solution turned very dark.
- Heat switched off and left stirring on the hot plate to slowly cool. A brown suspension soon appeared, and as it cooled the colour changed until it
was eventually lemon coloured:
  
- After 30 minutes of stirring at room temp the solution was filtered. The remainder was washed in the funnel and left on the bench in an evaporating
dish overnight. The next morning it was partially dried:

- It was then placed under a steel dish in the sun for 4 hours.
- Final recovery was 6.5g of lime green powder. I don't know whether it is iron (ii) or iron (iii) so did not write a formula on the label:

- Cleaning the evaporating dish with concentrated HCl shows the product to dissolved in the acid with reasonable ease giving a yellow solution,
probably FeCl3?

Next will be nickel.
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
I wonder why in the world the color changed so drastically with the iron compound; is it thermochromic? Have you tried gently heating it to see if it
becomes a darker brown again?
Also, since picolinic acid is an isomer of nicotinic acid (niacin) and isonicotinic acid, I'm really curious whether or not these also have vivid
salts or complexes. Anyway, great work, I hope we get to see more of these.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Hi Amos I'll heat some of the iron 'picolinate' on a steam bath and see what happens....next time in the shed.
I'll see if nicotinic acid is readily available.
Nickel:
- 10g of zinc picolinate dissolved in near boiling water, giving the usual completely clear solution (after some 15 minutes stirring).
- 9.5g nickel nitrate (lovely green crystals) dissolved in 25g water, giving a deep green solution.
- Nickel nitrate poured into hot zinc picolinate while stirring. The solution immediately turned a beautiful blue:

- No suspension appeared, and the heat was switched and the solution stirred while cooling. A lighter blue suspension soon appeared. It maintained the
same color when at room temperature.

- Filter solution at room temp. The remainder was baby blue, and the filtrate more "electric blue". This surprised me as I expected a slightly green
filtrate from excess nickel nitrate.

- The remainder was transferred to a evaporating dish for drying:

- After standing overnight on the bench (where it lost a gram in weight as it dried) and then some 6 hours under a steel dish in the sun the product
seemed dry. It is soft and easy to break up.

- 10g was bottled:

* The leftovers in the dish dissolves easy in concentrated HCl to give a green solution.
* If I assume the below equation, thus assuming my zinc picolinate is anhydrous, it goes along with nearly all the nickel nitrate being consumed (and
the lack of green filtrate):
Zn(C6H4O2N)2 + Ni(NO3)2.6H2O = Ni(C6H4O2N)2 + Zn(NO3)2 + H2O
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Hi Amos nicotinic acid is very readily available but I am confused - seems nicotinic and picolinic acids have the same formula, C6H5NO2?
Edit: I see now - same formula but different structure. I'll order some, it will indeed be interesting to see if the same colours appear.
[Edited on 2-8-2021 by Lion850]
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
I think the carboxylate being adjacent to the amine's lone pair allows for different complexes to form, but how this works and its effect on color are
beyond me.
Reflux condenser?? I barely know her!
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Hi njl - I have ordered nicotinic acid, will try once received! Online I saw a photo of chromium nicotinate and it looks very different from chromium
picolinate.
To conclude the run of making picolinates:
I tried a double displacement between neodymium nitrate and zinc picolinate but nothing happened. There was not reaction and the zinc picolinate
simply fell out of solution again upon cooling.
For now, this is the end of making picolinates. It was fun, I think good experiments for beginners, easy and yields interesting colours. Below is a
family photo of my picolinic salts; of the course the zinc picolinate was not made by me, it is the leftover from my purchase.

|
|
|