ChemTalk
Hazard to Self
 
Posts: 65
Registered: 13-12-2018
Location: United States
Member Is Offline
Mood: colloidal
|
|
Formation of red Samarium (II) Chloride
We made a short 60-second video showing the formation of the rarely seen red samarium (II) chloride, along with some close-ups of elemental samarium
taken with our new macro lens.
We hope you enjoy, let us know how you like it, and if there are any other rare-earth element reactions or elements you would be interested in seeing.
https://www.youtube.com/watch?v=BjBWzd04Qzo
Scott
PS -we heard that samarium (II) sulfate may be even more stable, but we have not been able to produce it yet.
|
|
|
Bedlasky
International Hazard
    
Posts: 1244
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Nice video! If you have THF and iodine, you can make stable solution of SmI2, which is red. You can also make green solution of YbI2 in the same way.
Here are some materials:
https://books.google.cz/books?id=QUfQamGsEgwC&printsec=f...
https://pubs.acs.org/doi/10.1021/ja00528a029
Ce(IV) form red complex [CeCl6]2- in conc. HCl (and also chlorine and Ce3+). Heating cause full decomposition to Ce3+ and Cl2.
I read something about Pr(IV) and Tb(IV) complexes with periodate, tellurate and phosphotungstate, which may be interesting and very strong oxidants.
|
|
|
ChemTalk
Hazard to Self
 
Posts: 65
Registered: 13-12-2018
Location: United States
Member Is Offline
Mood: colloidal
|
|
Bedlasky, thanks for the nice comment, and for the suggestions. We'll try to get some THF. I wonder if it would work in Xylene?
I have several rare earth elements, I have to check to see if I have Ytterbium, I might. we have Cerium (IV). Thanks for the links.
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
It isn't possible for xylene. Look at the photo, in solution samarium iodide is connected with five molecules of THF.
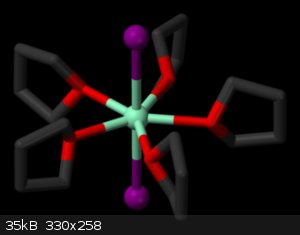
|
|
|
Bedlasky
International Hazard
    
Posts: 1244
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
ChemTalk: Other solvents are possible according that book. There are examples of other solvents: HMPA, acetonitrile, isopropyl alcohol, tert-butyl
alcohol, heptan-2-ol and DME (but I honestly don't know what they mean by DME, there are several compounds with this abbreviation). It is important
that solvent can coordinate to Sm(II).
[Edited on 16-7-2021 by Bedlasky]
[Edited on 16-7-2021 by Bedlasky]
|
|
|
ChemTalk
Hazard to Self
 
Posts: 65
Registered: 13-12-2018
Location: United States
Member Is Offline
Mood: colloidal
|
|
Bedlasky,
Thanks. It is easy to get THF here, they actually sell it on Amazon.
|
|
|
nezza
Hazard to Others
  
Posts: 324
Registered: 17-4-2011
Location: UK
Member Is Offline
Mood: phosphorescent
|
|
Here's my video of Samarium dissolving in dilute hydrochloric acid.
Attachment: Samarium-1.mp4 (3.8MB)
This file has been downloaded 245 times
If you're not part of the solution, you're part of the precipitate.
|
|
|
ChemTalk
Hazard to Self
 
Posts: 65
Registered: 13-12-2018
Location: United States
Member Is Offline
Mood: colloidal
|
|
thanks Nezza, this is a great video. I think you posted this some time ago? I feel like I've seen it before
You got much more samarium (II) chloride, yours was much more red. I'm not sure why, maybe your hydrochloric acid was more dilute? Or your samarium
piece was larger than mine?
|
|
|