garphield
Hazard to Self
 
Posts: 58
Registered: 9-12-2019
Member Is Offline
|
|
Reaction of sulfur and TCCA
Note: I am going to use sulfur chlorides to refer to SCl2 and/or S2Cl2, since both are interchangeable for many applications and are often formed as a
mixture.
I am currently working on a possible synthesis of phosphoryl chloride from entirely OTC materials that requires sulfur chlorides as an intermediate. I
have made sulfur chlorides in the past, but this is fairly difficult as due to not having as much glassware as I would like, I cannot distill the
sulfur chlorides as they form, but instead have to melt sulfur, let it cool down while bubbling chlorine gas through it, then disassemble the
apparatus and set it up for distillation.
If you could make it via simply adding TCCA to molten sulfur, it would be much easier to prepare. I could find one thread that went over this in some detail. It seems like the reaction of TCCA with sulfur in methylsulfonylmethane did not produce much sulfur
chlorides, although the solvent may have interfered with the reaction. However, another poster said he heated a mixture of TCCA, NaCl, and sulfur
without a solvent and got some drops of liquid and the smell of sulfur chlorides (he said he had made them before and remembered the smell, and as
someone who has smelled them it is a uniquely awful and characteristic smell so at least some was probably generated). However, it seems like that guy
might have messed up on the stoichiometry, and I'm not sure if the NaCl even did anything since any Na cyanate, thiocyanate, ect. salts that were
generated would most likely react with the sulfur chlorides to form sodium chloride and other stuff.
The question is, what exactly is that other stuff? I legitimately can't figure this out. It seems almost certain to me that the chlorine from the TCCA
would go to forming sulfur chlorides, but that would leave (OCN)3. Neither that molecule, or (OCN)2 (as an oxygen analogue of thiocyanogen) seem to
exist. Can anyone come up with any ideas of where the oxygen, carbon, and nitrogen might go? I might try this on a small (<1g) scale, but I would
still be kind of worried since it might produce cyanogen, carbonyl diisocyanate, or other very nasty compounds like disulfur dithiocyanate.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Nacl + sulfur being heated together apparently yeild scl2 by itself
without the tcca
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
To whom is this apparent?
In any event, remember that you are proposing to heat a strong oxidant with a good fuel.
[Edited on 26-7-21 by unionised]
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
NaCl + sulfur? I think that no reaction occurs at all, even when heated so much that the sulfur boils off as gas.
|
|
|
teodor
National Hazard
   
Posts: 922
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
ZnCl2 and FeCl3 have their melting point below sulfur, if this route would work these salts (especially the ferric one) should be a good alternative
to gaseous Cl2. But I never saw any references to such reactions in literature.
ZnCl2 is possible to get in anhydrous form starting from OTC materials.
If it works we should expect the possible reaction of FeCl3 with P2O5, but I wouldn't try to go this way in case of limited glassware.
But be aware of danger of these molten chlorides, their vapours could be very toxic.
I believe that S2Cl2 is also quite toxic in form of vapours, OSHA PEL has 1 ppm for S2Cl2 and 10 ppm for cyanogen, but not sure they are practically
comparable in amateur environment.
I think that the reaction of molten sulfur with gaseous Cl2 which forms S2Cl2 as a liquid (not as a gas) is more amateur-friendly (see Brauer for the
details of the preparation).
[Edited on 27-7-2021 by teodor]
[Edited on 27-7-2021 by teodor]
Also about phosphoryl chloride from OTC, I hardly can imagine that somebody who has only OTC compounds and limited glassware can make something
interesting/good with this compound.
[Edited on 27-7-2021 by teodor]
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
While sulfuryl chloride is a good chlorinating agent, I cannot see sulfuryl chloride replacing oxygen in phosphorous pentoxide or phosphoric acid, and
phosphorous pentoxide is not exactly OTC, although it is occasionally for sale in small quantities.
phosphorous loves oxygen as much as I love popeye's.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2799
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
At least one SM member observed an explosion after attempting to distill a reaction mixture
containing TCCA and benzaldehyde. Attempting to drive a TCCA+S8 reaction by heating could easily meet the same fate:
2 C3N3O3Cl3 + S8 >> 3 S2Cl2 + S2 + 6 CO + 3 N2 + lots of kilojoules
This sounds incredibly unlikely, given that the boiling point of sulfur (disulfur gas forms around 450 C) is low enough that SCl2 formation would
never be entropically favorable. ZnCl2 will not react with sulfur.
This could happen:
8 FeCl3 + S8 >> 8 FeCl2 + 4 S2Cl2
However, the rxn onset temperature likely exceeds the autoignition temperature of sulfur (~200 C), which creates another problem!
|
|
|
teodor
National Hazard
   
Posts: 922
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
I don't think the idea of high-temp synthesis of SxCly is a good idea because chlorine in S-Cl bond is always looking a way to be replaced by oxygen
when reaction is performed in presence of air. For example, by passing not oxygen-free chlorine through liquid S2Cl2 we obtain a mixed compound of
sulfur/chlorine/oxygen. And it looks like there is no way to replace oxygen by chlorine, all possible reactions I remember are about replacement of
S-OH with S-Cl but never S=O (well, you probably can do it with help of elements like P but there are not so many such elements like P).
[Edited on 29-7-2021 by teodor]
|
|
|
wg48temp9
National Hazard
   
Posts: 786
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Apparently TCCA and NaDIC (the hydrate, personal experience) can decompose explosively when heated. I suspect heating either one with sulphur will
make it more explosive. Try say a gram first, outside and standing well back.
See the following thread http://www.sciencemadness.org/talk/viewthread.php?tid=80458
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
In my above post I used the word apparently because I have neither done this myself nor do I know anyone who has and this did not come from a
textbook. I've spent a day looking for where I've seen NaCl+ sulfur --> scl2+na2s and I cannot find it anywhere. Where I originally seen this I
can't remember. Googling gets no results. I'll keep looking for the source of this information.
|
|
|
Σldritch
Hazard to Others
  
Posts: 310
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
I came across this thread while looking for a simple way to make Cyanuric Chloride. The idea i had was the following idealized reaction:
2 C3O3N3Cl3 + 3 S = 2 N3C3Cl3 + 3 SO2
I imagine the mechanism might be something like this:
1. Sulfur atom inserts inbetween chlorine and oxygen atom of TCCA oxidizing to +(II) state.
2. Cl-S-O- group swings on carbon atom over to the chlorine atom on the other side oxidizing sulfur to +(IV) state with negative charge delocalized on
ring and positive on sulfur.
3. Lewis base B forms adduct with the Sulfur atom displacing Chloride anion.
4. Chloride anion attacks C-O-S bond freeing SOCl:B+ leaving C-Cl.
5... SOCl:B+ acts like thionyl chloride completing the reaction producing SO2 and regenerating B.
Surely this is more plausible, and perhaps even more useful aswell. Not sure which lewis base would be suitable though. Ammonia is a no-go, pyridine
or dioxane is apparently used for Thionyl Chloride but they might get attacked by TCCA too. I can't try it anyway, my TCCA is full of crap.
As for SCl2, is it not easier to just make S2Cl2 and then use your TCCA to bubble Chlorine through your non-degrading
premade S2Cl2 in situ?
I guess you would get S2Cl2 from Sulfur and metal chlorides due to the temperature presumably required so it seems inevitable
anyway. I do see that reaction working though, makes sense volatility wise. Perhaps PbCl2 would be a good salt to use. IIRC it is a
byproduct from forming NaOH from NaCl and PbO even. PbS is very stable too, should be favorable to form.
EDIT:
I am an idiot: obviously the best lewis base to use is Chloride for the reaction i had envisaged.
[Edited on 7-6-2022 by Σldritch]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2799
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
I reiterate my concern that the risk of explosion has not been adequately addressed. Sulfur will not typically reduce a urea, so you need some kind of
fortuitous mechanism to attach those oxygen atoms to the sulfur.
EDIT: I read your proposed mechanism. You seem to be under the impression that there is an O-Cl bond. There is not; TCCA is
O=C1N(Cl)C(=O)N(Cl)C(=O)N1Cl
If you want to heat something with sulfur, my suggestion would be CuBr2, which begins to decompose around 160-200 C, where sulfur would be molten, and
S2Br2 exists above this temperature. I also considered the reaction with CuCl2, but thermodynamical calculations put the reaction temperature at 440
C, which is well above sulfur's autoignition point of 260 C. Online sources suggest that the ambient pressure bp of S2Br2 is 137 C.
[Edited on 7-6-2022 by clearly_not_atara]
|
|
|
Σldritch
Hazard to Others
  
Posts: 310
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
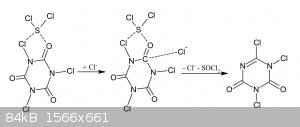
I think you misunderstood what i meant, perhaps i should have gone with a picture instead but i was lazy. Anyway having contemplated the mechanism
with Chloride as a lewis base i think i can simplify it a bit.
As you can see the somewhat positively charged sulfur atom of SCl2 (or perhaps S2Cl2 or even S8) is
attracted by the somewhat negative oxygen atom of TCCA. From there you get oxidation by the chloramine group and attack of Chloride on the carbonyl
carbon atom forming a Cyanuric Chloride and Thionyl Chloride. This may be concerted, or the relatively basic Cl2SO-group is ionized and Chloride freed
that way for attack on carbon. In the latter case the reaction may be catalyzed by Chloride.
If SCl2 is of importance the equilibria of the type below should be too:
S + TCCA + Cl-1 = SCl2 + DCCA-1
But perhaps the reaction should be seen as that between SCl2 and TCCA:
3 SCl2 + O3C3N3Cl3 = 3 SOCl2 + N3C3Cl3
That would be quite the useful reaction. It kind of makes sense from the view of SCl4 though. SCl4 forms in SCl2 with
excess Chlorine presumably. Normally the concetration would be limited by the volatility of Chlorine but TCCA acting as solid Chlorine should be able
to push it to one side. Now factor in that TCCA is a Cl+1 source - a much more energetic oxidizer - and that in this case we can go
straight to the more favorable Thionyl Chloride. In that light i think this mechanism is plausible and perhaps the reaction does occur.
|
|
|