| Pages:
1
2 |
Monoamine
Hazard to Others
  
Posts: 168
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
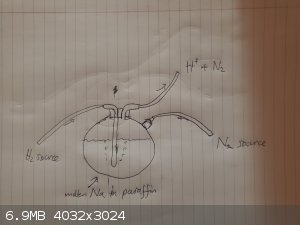
Preparation of NaH from sodium metal.
(Disclaimer: Elemental Na as well as NaH are highly reactive in air and can spontaneously ignite violently, proper safety
procedures are essential when attempting this!))
Sodium hydride (NaH) is a very strong base, which is strong enough to deprotonate alcohols, H20, and even the Indole nitrogen. It can also be used to
create other bases such as NaOCH2(CH3), which have as many useful applications.
I hope to be able to try to make it at some point.
The standard procedure for the preparation of NaH (according to Wikipedia) is to react molten Na metal with H2 gas. Na melts at 97.81o)C.
Na is often stored in Kerosine mineral oil (paraffin), which distills at 150oC to 300oC. In other words, the Na should be easily
molten when still stored in paraffin.
Once the Na has melted, we can begin pubbling H2 gas through the solution (this is probably the most dangerous step, since H2 is quite flammable). The
melting point of NaH is 638oC, so it should precipitate out solution as it is formed. The reaction is continued until no more NaH is
formed. The NaH is then stored directly in the paraffin, since this is the safest way of storing it.
As an extra precaution, it may be a good idea to continuously inject N2 gas into the top of the reaction flask to minimize the risk of combustions.
Also, as the reaction produces H+, this will have to be taken care of. This is the part I'm the least sure about... one method would be to
simply vent it into the fume hood, or pass it through a water bath, which should drop its pH.
I'd be curious to hear if anyone has any ideas about a good way to get rid of the H+ formed.
Below is the setup that could be used:
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Uh what?
You can deprotonate the indolic nitrogen even with plain lye if you just use a PTC 
Is great for the production of N-1 alkylindoles, just melt the indole in lye with a PTC and the alkyl halide, then let it cool down after half an hour
and you have an almost quantitative yield of N-alkylindoles.
|
|
|
Bedlasky
International Hazard
    
Posts: 1242
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
H+? What H+?
2Na + H2 --> 2NaH
And why do you talk about H+ as it is a gas? H+ practically even don't exist, because it binds to some electron donor.
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
I was more familiar with sodium hydride's deprotonation of the 3 position on indoles. What is it structurally that allows N deprotonation over C
deprotonation?
The H+ claim is probably just from not seeing the whole picture. H2 is not split into H+ and H- here as I believe Monoamine is implying. Instead, both
hydrogens are reduced with 2 electrons from 2 different sodium atoms.
edit: the exact details of this sort of reaction mechanism is the kind of thing people spend their careers studying so to be clear this is more of a
helpful way to think of things and not a completely accurate depiction of reality.
[Edited on 6-24-2021 by njl]
Reflux condenser?? I barely know her!
|
|
|
Monoamine
Hazard to Others
  
Posts: 168
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Quote: Originally posted by karlos³  |
Uh what?
You can deprotonate the indolic nitrogen even with plain lye if you just use a PTC 
Is great for the production of N-1 alkylindoles, just melt the indole in lye with a PTC and the alkyl halide, then let it cool down after half an hour
and you have an almost quantitative yield of N-alkylindoles. |
Good to know. Thanks!
|
|
|
Monoamine
Hazard to Others
  
Posts: 168
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Quote: Originally posted by njl  | I was more familiar with sodium hydride's deprotonation of the 3 position on indoles. What is it structurally that allows N deprotonation over C
deprotonation?
The H+ claim is probably just from not seeing the whole picture. H2 is not split into H+ and H- here as I believe Monoamine is implying. Instead, both
hydrogens are reduced with 2 electrons from 2 different sodium atoms.
edit: the exact details of this sort of reaction mechanism is the kind of thing people spend their careers studying so to be clear this is more of a
helpful way to think of things and not a completely accurate depiction of reality.
[Edited on 6-24-2021 by njl] |
For the N-deprotonation, I would have thought that since the H on the indole N is in an sp2 orbital rather than a pi orbital it should be more easily
deprotonated (I think you don't destroy aromaticity of the indole ring by plucking a proton from the N, since it's the lone pair that's in the pi
orbital?)
But you're saying one can deprotonate the 3-proton on indole? I had no clue this was even possible! Very interesting. Now I'm starting to wonder what
reaction this could enable.
But indole aside, it would certainly also be useful for deprotonating OH groups and making some nice sterically hindered bases and such
Also thank you for clarifying the proposed mechanism for how NaH is formed! Much appreciated! In that case I guess all you would have to do is vent
the N2 and the unreacted H2 out safely somehow. (The H2 to be precise, not so worried about the N2).
[Edited on 25-6-2021 by Monoamine]
|
|
|
rockyit98
Hazard to Others
  
Posts: 283
Registered: 12-4-2019
Location: The Known Universe
Member Is Offline
Mood: no mood is a good mood
|
|
Sodium react with hydrogen to produce sodium hydride. This reaction takes place at a temperature near 300 ° C.
needs catalysts. maybe naphthalene.
"A mind is a terrible thing to lose"-Meisner
|
|
|
Monoamine
Hazard to Others
  
Posts: 168
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Quote: Originally posted by rockyit98  | Sodium react with hydrogen to produce sodium hydride. This reaction takes place at a temperature near 300 ° C.
needs catalysts. maybe naphthalene. |
Not too familiar with catalysts. Why is naphthalene a catalyst for this reaction?
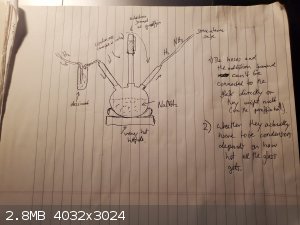
Btw, I think what you're referring to is this article:
Guntz; Benoit; Bulletin de la Societe Chimique de France; vol. 41; (1927); p. 434 - 434
There they used sodium amide which was heated in a H2 atmosphere until the NaNH2 decomposed, which gave NaH. Unfortunately I can't find the
actual paper online, but it seems a little dangerous tbh...
I'm guessing the reaction is NaNH2 + H2 -> NaH + NH3\, so you'll have to drive off the ammonia as it forms (maybe by
`crowding it out' by just pumping in H2 continuously. And of course this has to be done in completely anhydrous conditions. So drying tubes on the way
in and out are a must. And, you'll probably want a way to pour in paraffin to cover the NaH you made once it's cool enough (but keep the H2 running,
or switch to something more inert so no nasty water seeps in....)
I guess you would know the reaction is done once no more NH3 is produced...
But.... it might be doable... if you're careful enough... Probably wanna hide behind a blast shield or something...
|
|
|
Keras
National Hazard
   
Posts: 931
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
There’s a chapter about that in the book Small Scale Synthesis of Laboratory Reagents
[Edited on 25-6-2021 by Keras]
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
http://www.sciencemadness.org/smwiki/index.php/Sodium_hydrid...
|
|
|
Bedlasky
International Hazard
    
Posts: 1242
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Quote: Originally posted by Monoamine  | Quote: Originally posted by rockyit98  | Sodium react with hydrogen to produce sodium hydride. This reaction takes place at a temperature near 300 ° C.
needs catalysts. maybe naphthalene. |
Not too familiar with catalysts. Why is naphthalene a catalyst for this reaction? |
Sodium react with naphtalene to form sodium naphtenide, which than react with hydrogen gas to produce NaH.
2Na + 2C10H8 --> 2NaC10H7 + H2
NaC10H7 + H2 --> NaH + C10H8
|
|
|
rockyit98
Hazard to Others
  
Posts: 283
Registered: 12-4-2019
Location: The Known Universe
Member Is Offline
Mood: no mood is a good mood
|
|
water free NaOH react with Na metal to form NaH and Na2O but at high pressures .how much idk. might worth checking out.
"A mind is a terrible thing to lose"-Meisner
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Not the way I would do it. I would prefer to make it by the pound, via a stirred pressure reactor, but...to each his own.
From Len1's book.
6.3 exPerIMental
6.3.1 Sodium hydride
A quartz test tube about 30 mm in diameter and at least 200 mm long is placed inside a sealed tube oven or a circular opening in a box oven, and
inclined upwards at 5°–8° to the horizontal. About 3–4 g of sodium metal, which has been shaken under ether to remove the last traces of
paraffin, is placed inside a stainless steel tube, closed at one end, and inserted into the quartz reactor. A measured amount of steel wool at the
bottom of the quartz test tube is used to adjust the location of the steel tube opening so it lies just inside the oven at the point where a
temperature gradient is expected to commence. A side arm on the quartz tube leads to a U-tube containing several sections filled with P2O5 and
separated by glass wool. The other end of the U-tube is connected to a hydrogen cylinder through a flow-rate adjusting valve. A long-arm stainless
steel spatula (which can be fashioned out of a thin- diameter S/S pipe) is inserted through a seal so that its end lies just outside the zone where
NaH is expected to be formed. The spatula runs through a fairly long section of straight tube prior to reaching the active zone in order to minimize
the angular movement of the spatula and disturbance to the seal when removing the product. The outlet gases from the reactor are passed through an
empty washbottle and an oil bubbler, which both isolates the reactor and serves as an indicator of the pres- sure inside.
The air inside the reactor is purged by opening the hydrogen valve until no oxygen is evident in the outlet gas, and the oven temperature is raised in
the range 610°C– 640°C (corresponding to a tube temperature of about 550°C–580°C). Hydrogen absorption commences at about 570°C as evidenced
by a slow rise of oil inside the washbottle capillary, and the hydrogen valve is opened so that the level in the capil- lary remains about constant.
There is some nonuniformity in hydrogen absorption with time, and the hydrogen feed rate should be adjusted on the high-side, which leads to some loss
of hydrogen. Alternatively, a hydrogen balloon can be used.
The sodium hydride starts forming immediately outside the tube opening where the local temperature is below its decomposition temperature at 1 atm
hydrogen pressure. Figure 6.2 shows the result after about 20 min of operation. After about 2 h, a wool-like plug of sodium hydride needles completely
occupies the temperature region suitable for hydride formation and hydrogen absorption slows. The hydrogen flow rate can be increased at this point to
produce positive pressure inside the reac- tor, and the spatula can be inserted into the active region and rotated to remove the plug into the
low-temperature region of the reactor where the hydride is stable. This operation is repeated every few hours, resulting in an NaH formation rate of
about 0.2–0.3 g/h. When sufficient NaH has formed, the quartz tube is withdrawn from the reactor and allowed to cool to near room temperature. At
that point, the hermi- ticity of the apparatus can be broken, and the sodium hydride removed in the open atmosphere. The author has found that no
spontaneously ignitable sublimates form in the reaction.
Raising the reactor temperature above about 640°C does not lead to an increased rate of hydride formation; rather, a gray color appears in the
product corresponding
© 2011 by Taylor and Francis Group, LLC
78 Small-Scale Synthesis of Laboratory Reagents with Reaction Modeling
to condensed unreacted sodium. Higher temperatures still lead to decomposition of the hydride already formed and its reformation in the section of the
reactor, which now has the appropriate temperature. However, the higher evaporation rate also leads to sodium globules forming in the NaH matrix as
well as condensation of liq- uid sodium on the walls of the quartz reactor. This is deleterious to the quartz tube because of the danger of liquid
sodium flowing into the high temperature region and reducing the quartz in depth. Gaseous sodium on the other hand does not seriously attack quartz,
producing just a superficial discoloration, which disappears (due to the silicon being oxidized back to silica) on exposure to air.
LiH is purportedly easier to make?
[Edited on 26-6-2021 by zed]
|
|
|
rockyit98
Hazard to Others
  
Posts: 283
Registered: 12-4-2019
Location: The Known Universe
Member Is Offline
Mood: no mood is a good mood
|
|
Thoisoi2 - Chemical Experiments! uploaded: This Chemical is Not from Our Planet! shows how dangerous NaH is
"A mind is a terrible thing to lose"-Meisner
|
|
|
BromicAcid
International Hazard
    
Posts: 3253
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
When running a Castner Cell, NaH starts to form in the melt if the temp is too high.
|
|
|
Monoamine
Hazard to Others
  
Posts: 168
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Quote: Originally posted by Bedlasky  | Quote: Originally posted by Monoamine  | Quote: Originally posted by rockyit98  | Sodium react with hydrogen to produce sodium hydride. This reaction takes place at a temperature near 300 ° C.
needs catalysts. maybe naphthalene. |
Not too familiar with catalysts. Why is naphthalene a catalyst for this reaction? |
Sodium react with naphtalene to form sodium naphtenide, which than react with hydrogen gas to produce NaH.
2Na + 2C10H8 --> 2NaC10H7 + H2
NaC10H7 + H2 --> NaH + C10H8 |
Interesting! Thank's for sharing! So this should even be doable at 97.81oC (the melting point of Na)?
One question though: Once all the Na has reacted, there would still be the C10H8 catalyst left, right? I would guess that it's soluble in paraffin
oil, but does simply keeping it in there pose a problem or would you have to try to remove it somehow?
|
|
|
Monoamine
Hazard to Others
  
Posts: 168
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Quote: Originally posted by Keras  | There’s a chapter about that in the book Small Scale Synthesis of Laboratory Reagents
[Edited on 25-6-2021 by Keras] |
Thanks for the reference! I'll check out the PDF; always eager to learn!
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Read the literature, room temp in this case US3617218 and the JACS article from the same authors...
Brauer, Inorg Syn...
|
|
|
Monoamine
Hazard to Others
  
Posts: 168
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Quote: Originally posted by S.C. Wack  | Read the literature, room temp in this case US3617218 and the JACS article from the same authors...
Brauer, Inorg Syn... |
Well that does seem safer... but you probably still want to melt the Na first to make it into as tiny pebbles as possible to maximize surface area,
otherwise I'd imagine the reaction will take pretty long to finish.
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
How about this post on sodium metal on silica gel as a catalyst. This may help the absorbing of hydrogen gas. I've read somewhere that someone used
titanium powder to help it absorb the gas.
http://www.sciencemadness.org/talk/viewthread.php?tid=14626
[Edited on 28-6-2021 by symboom]
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
I think that Li reacts with the H2 around 60'c which is a bit easier than
molten metals.
[Edited on 28-6-2021 by draculic acid69]
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Put a 1way check valve on your output hose and vent into a candle to burn it off
Bubbling thru water won't do anything at all
[Edited on 28-6-2021 by draculic acid69]
|
|
|
cirice1
Harmless

Posts: 12
Registered: 8-1-2019
Member Is Online
|
|
https://pubs.acs.org/doi/abs/10.1021/ja01026a057
https://sci-hub.se/10.1055/sos-SD-008-00607
|
|
|
wg48temp9
National Hazard
   
Posts: 786
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by draculic acid69  |
Put a 1way check valve on your output hose and vent into a candle to burn it off
Bubbling thru water won't do anything at all
[Edited on 28-6-2021 by draculic acid69] |
Just in case the above is serious: to separate even a ug of H+ ions from their electrons for any significant distance (say 100mm) would require
hundreds of kj of energy and require overcoming huge forces. The voltage between the H+ ions and its electrons or ground would be millions of volts.
So its impossible to collect any significant mass of H+. If any H+ ions were formed in a reaction mixtures they would remain in the reaction mixture
to be near their electrons.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
500
The easiest route to hydrides (not in the literature as a preparative method AFAIK) is by heating RM (R2M or RMX for Mg, etc) in oil.
[Edited on 28-6-2021 by S.C. Wack]
|
|
|
| Pages:
1
2 |