Monoamine
Hazard to Others
  
Posts: 168
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Question about sulfonation of furan
I recently came across some lecture notes on sulfonic acid protection of furan to control its regiochemistry (Lecture notes).
From what I gather, electrophilic substitution tends to happen at the ortho positions of furan. So if we want to substitute the other positions we
need to add a protecting group.
In the lecture notes the following scheme was proposed (see attachment).
The problem, as usual, is that there are no details given as to how to actually do this. In particular, no information about the amount of sulfur
trioxide pyridine complex to be used, and for how long the reaction should run.
Does anyone know the details of how to do this reaction? And is there the risk of over sulfonating the furan ring?
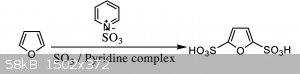
[Edited on 3-6-2021 by Monoamine]
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
Start here: DOI: 10.1021/jo01371a003
Gives prep of the Pyridine*SO3 reagent and 4 different preps of furan sulfonic acids.
|
|
|
Monoamine
Hazard to Others
  
Posts: 168
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Quote: Originally posted by UC235  | Start here: DOI: 10.1021/jo01371a003
Gives prep of the Pyridine*SO3 reagent and 4 different preps of furan sulfonic acids. |
Awesome thanks for the reference!!
Not quite sure about how they purify the target compound, 2,5-furan disulfonic acid though...
It probably has a higher boiling point than furan and 2-furansulfonic acid, so the by-products could be distilled of from the target compound.
Also, I wonder if adding a dean stark trap filled with desiccant and a drying tube over the reflux condenser would not eventually drive the reaction
to completion (if the reaction is run longer), since all the water would be removed, thus preventing the reaction to be reversible. This would then
also negate the need to purify the various sulfonated furan species.
The di-sulfonated furan can then just be filtered off from the sulfonating agent.
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
You won't find a much better desiccant than SO3.
|
|
|
Boffis
International Hazard
    
Posts: 1883
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I don't know what your motivation for looking at furan sulphonic acids is but there is another route into substituted furan sulphonic acids via so
called endialone. Furfuraldehyde is oxidized by a restricted supply of aqueous chlorine to "endialone solution" and then reduced again by sodium
sulphite to furfuraldehyde-5-sulphonic acid.
If you are interested I can dig out some references.
|
|
|