Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Making silicon tetrachloride
I want to make silicon tetrachloride, but don't want to do the traditional method of heating silicon with chlorine. Instead, I was thinking that
reacting silicon with iodine at ~180C under sulfuric acid would create silicon tetraiodide, and reacting that with chlorine gas to give the silicon
tetrachloride. Would this work?
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Look at this:
http://www.sciencemadness.org/talk/viewthread.php?tid=156987...
It works on gallium, but also on antimony. I was surprised when I really made antimony triiodide with this reaction. I think it is worth a try. If you
can, once use hydrochloric acid instead of sulfuric acid, I wonder if it will work on silicon.
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
The sulfuric acid is just to keep the iodine from sublimating into oblivion, no actual reaction.
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Of course Yes, but i don't think that it will stop sublimation(At least at the required level) . What percentage of sulfuric acid are you going to
use?
[Edited on 9-5-2021 by vano]
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Around 98%, don't have anything stronger. Why won't it stop the sublimation, I've seen people melting and cast iodine under sulfuric acid.
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Good percentage, maybe it's possible. I thought that you have low percentage acid, because 98% is hard to find in many countries.
Okey, i like you idea. Do you have silicon powder?
Different is that you can dissolve iodine in HCl, i will try this reaction.
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
I think halosilicates are also interesting. I wonder if this is possible on chlorine. Second is easiest i think.
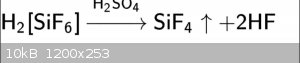


|
|
|
Bedlasky
International Hazard
    
Posts: 1243
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Triflic acid: You can try it. Maybe it work? I really don't know, I just know classical ways like Si + Cl2 or SiO2 + C + Cl2.
Vano: SiCl4 cannot be made so easily like SbI3 or GaI3. Any presence of water will lead to hydrolysis of SiI4 (but I doubt that you can make SiO2 by
this way).
Silicon form just hexafluorosilicates, similar chloro complexes doesn't exist.
|
|
|
wg48temp9
National Hazard
   
Posts: 786
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Perhaps the silicon tetrachloride could be made from alkoxysilanes which can be made by dissolving silica in ethylene glycol,
see:
PS: How do I link to a previously posted paper so I don't have to upload it again and how do I reference a previous post without quoting it?
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Bedlasky:I remembered that calcium hexachlorosilicate exist.
|
|
|
Bedlasky
International Hazard
    
Posts: 1243
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Source?
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
You will not successfully convert Si(OR)4 to SiCl4, even if you can obtain the former; the affinity of silicon for oxygen is too high. You may be able
to do it with SiF4 but the equipment for handling SiF4 is not any simpler than the equipment for making SiCl4 by the usual direct process.
I don't know about the iodine/sulfuric acid method. It seems possible that all of the following reactions occur in that system:
Si + I2 >> SiI4
SiI4 + 2H2SO4 >> SiO2 + 2SO3 + 4 HI
SO3 + 2 HI >> SO2 + H2O + I2
leading to a net reaction like this:
Si + 2 H2SO4 [cat I2] >> SiO2 + 2 SO2 + 2 H2O
which is, as you have guessed, completely useless. Silicon halides are extremely reactive. They will not form easily and they react with practically
any hydroxyl available.
The direct chlorination of metal silicides is preferred for a reason... they react at low temperatures and give no byproducts. Mg2Si can be made by
"careful" thermite-like reaction. It can probably be brominated at r.t. as well.
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Would SiI4 really dehydrate sulfuric acid  I have 100g of silicon lumps coming
in the mail today. Should I try to get some magnesium silicide as well and try that? Also, if not H2SO4, can you think of a different high boiling
solvent with no hydroxyls? I have 100g of silicon lumps coming
in the mail today. Should I try to get some magnesium silicide as well and try that? Also, if not H2SO4, can you think of a different high boiling
solvent with no hydroxyls?
[Edit: If it does dehydrate I'm screwed: https://www.jstor.org/stable/20022852?seq=1#metadata_info_tab_contents]
[Edited on 10-5-2021 by Triflic Acid]
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
bump
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Could you use ferrosilicon instead of trying to make magnesium or aluminium silicide. It is easily available off Ebay. You would still need hot
chlorine gas but the fine powder may react faster than silicon itself. Let us know how you get on making magnesium silicide; its usually made by
reaction dry silica with excess magnesium in a thermite like reaction. Silicon is not very reactive and the mixed element powder will have a lot of
void space between the particle full of air so oxide and nitride films are likely to make the reaction difficult unless you can flush or mix the
powders in argon first.
|
|
|
rockyit98
Hazard to Others
  
Posts: 283
Registered: 12-4-2019
Location: The Known Universe
Member Is Offline
Mood: no mood is a good mood
|
|
one way of making TiCl4 is heating sodium pyrosulfate with NaCl and TiO2. ( 2Na2S2O7 + 4NaCl +TiO2 -----(500C or more)----> TiCl4(g) +4Na2SO4 )
maybe replacing TiO2 with SiO2 will make SiO2 gas.
"A mind is a terrible thing to lose"-Meisner
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Update, the prep I’m following calls for teos so I’m going to make that directly instead of making silicon tetra chloride. There is a new thread
on that, since it is unrelated to this one.
[Edit: that thread is in organic, http://www.sciencemadness.org/talk/viewthread.php?tid=157461]
[Edited on 19-5-2021 by Triflic Acid]
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
Shenno
Harmless

Posts: 4
Registered: 27-6-2019
Member Is Offline
|
|
i just wanted to point out that silicon tetrachloride is an evil compound and extremely volatile .. upon my own experience ( i used the chloride +
silicon route) it creates pressure inside the storage vessel even if you kept it dissolved in a hydrocarbon. so be very careful while handling this
compound
i never had any good yields from my impure silicon powder .. i also tried an indirect route through silicon sulfide which was another pain in the ass
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Welcome to the forum, and thanks for the warning. Also, how did you make the silicon sulfide? I’m trying to make some myself.
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
Shenno
Harmless

Posts: 4
Registered: 27-6-2019
Member Is Offline
|
|
Thanks Triflic Acid
i prepared silicon disulfide through the elemental route + 0.5 mole precipitated silica for every mole of silicon, it seemed to work well but for some
reason only 25 % of the mass was reactive when i added anhydrous ethanol
this is the link of the reaction .. i ignited it with magnesium thermite which i ignited with KMnO4 and Glycerin
https://1drv.ms/v/s!Ao-xsE8BnvftmQPgO7y3DdMwfUQq?e=l4tvse
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
You mean precipitated sulfur, right?
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
Shenno
Harmless

Posts: 4
Registered: 27-6-2019
Member Is Offline
|
|
silicon 100 micron particles + precipitated sulfur + precipitated silica
Ref: US2766103
|
|
|