| Pages:
1
2 |
Axt
National Hazard
   
Posts: 817
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Glyoxime, Diaminofurazan and Some Energetic Derivatives
<center><font size="6">Glyoxime, Diaminofurazan</font>
<font size="4">and some Energetic Derivatives</font></center>
<font size="5">Hydroxylamine Hydrochloride</font>
<img src="http://www.sciencemadness.org/scipics/axt/hydroxylamine.jpg">
Hydroxylamine can be formed by acid hydrolysis of nitromethane[1] forming the acid salt of hydroxylamine and formic acid.
CH<sub>3</sub>NO<sub>2</sub> + HCl + H<sub>2</sub>O → NH<sub>2</sub>OH.HCl + HCOOH
Hydroxylamine hydrochloride: 61g nitromethane was mixed with 114g 32% hydrochloric acid in a 300ml glass bottle (molar ratio CH3NO2/HCl/H2O 1:1:4.3).
The top was screwed on the bottle and it was immersed in an oil bath heated to 100°C (figure 1). The solution was left at this temperature for 24
hours whereby the nitromethane and acid layers formed a homogeneous solution.
<center><img src="http://www.sciencemadness.org/scipics/axt/hydroxylamine-syn.jpg">
<i>Figure 1: CH3NO2/HCl solution in oil bath (left). NH2OH.HCl crystals (right)</i></center>
The solution was then transfered to a wide mouth beaker and left at the same temperature until evaporated to about 1/3 of its initial volume. On
cooling the solution to -5°C hydroxylamine hydrochloride precipitated as large white flakes which were filtered and dried, further concentration and
cooling yielded more crystals for a total of 41g (59%).
A similar process to that described above was used but with a molar ratio of 1:1:10, this allowed the solution to be heated in a non-pressurized
vessel without significant loss of HCl, plastic film and a rubber band was used to cover the flask containing the solution. The solution was heated
for 40 hours at 100°C and concentrated and precipitated as before, yield was 32g (46%).
When heated on a spoon the NH2OH.HCl decomposed energetically with release of white smoke but no flame.
<font size="5">Glyoxime</font>
<img src="http://www.sciencemadness.org/scipics/axt/glyoxime.jpg">
Glyoxime also known as ethandial dioxime is produced by the condensation of glyoxal with hydroxylamine in slightly acidic solution, the following is
an adaptation of the literature method[17].
Glyoxime: 27.5g (0.69mol) sodium hydroxide was dissolved into 75ml water and cooled to 0°C, 69.5g hydroxylamine hydrochloride (1mol) was then added
with stirring. To the still chilled solution was then slowly added 72.5g (0.5mol) of 40% glyoxal in 48ml water maintaining temperature below 10°C.
The solution was left in the fridge for 15 minutes then removed and allowed to come to room temperature. The solution become solid with precipitate
within 1 hour which was scooped out and pressed as dry as possible between absorbent paper, The damp glyoxime was then left sandwiched between 4
sheets of absorbent paper to dry (figure 2), this allowed the salt solution to be wicked out of the glyoxime to deposit the NaCl in the upper layers
of paper. The crude yield of glyoxime was 38g (86%). The crude glyoxime was recrystalised from ether to form the most pure product, this was used for
the lead and silver salts below.
<center><img src="http://www.sciencemadness.org/scipics/axt/glyoxime-dry.jpg">
<i>Figure 2: Drying glyoxime</i></center>
The recrystalised glyoxime melts at 178°C with decomposition and ignites easily when touched with the flame of a match, burning mildly with a soft
orange flame and faint “hiss”.
<font size="4">Glyoximate Salts</font>
Glyoxime is acidic, and carries the energetic oxime groups, thus is capable of forming salts that may show notable explosive characteristics.
Bretherick[2] lists a basic copper salt (HOCuON=CHCH=NOH) which is said to lose weight up to 140°C before exploding.
The silver and lead salts were produced by precipitation of a solution of sodium glyoximate with silver nitrate and lead acetate. These salts were
shown to have interesting primary explosive qualities but are unlikely to have, nor were they tested for initiating ability.
Silver glyoximate: Into 2g (23mmol) of glyoxime in 50ml water was added 1.8g (45mmol) of sodium hydroxide to produce a solution of sodium glyoximate.
Into this solution was added 3.9g (46mmol) of silver nitrate in 20ml water which resulted in the immediate precipitation of a grayish maroon coloured
silver glyoximate, which was filtered and dried out of sunlight. The dried salt was found to darken when exposed to sunlight and flash with a
“thump” on ignition (figure 3). Eventually the silver salt exploded by itself when left in direct sunlight.
Lead glyoximate: Lead glyoximate was produced in the same way as the silver salt, except 7.4g (23mmol) of lead acetate was used to precipitate the
lead glyoximate as a fine, flocculent white precipitate. The lead salt on ignition exploded with more vehemence then the silver salt, exploding with a
loud report in quantities over about 1g (figure 3).
<center><img src="http://www.sciencemadness.org/scipics/axt/glyoximates.jpg">
<i>Figure 3: Ignition of 0.8g lead glyoximate (top) and 0.5g silver glyoximate (bottom)</i></center>
<font size="5">Nitroglyoxime</font>
<img src="http://www.sciencemadness.org/scipics/axt/nitroglyoxime.jpg">
The activated hydrogen of glyoxime is capable of reacting with nitric acid containing HNO2[3] or with nitrogen dioxide itself[4] yielding
nitroglyoxime, an explosive nitrolic acid.
Nitroglyoxime is too unstable to be of any practical use, it melts at 111°C with decomposition and explodes on further heating[4]. It is soluble in
water (decomposed by hot water) alcohol, ether and acetone, very slightly soluble in benzene, more so in boiling benzene. It is insoluble in
chloroform and petroleum ether[4].
The basic lead salt of nitroglyoxime (C2H2N3O4.Pb.O.Pb.C2H2N3O4) was first reported by Bamberger[4] as being the yellow precipitate that forms from
combining aqueous solutions of nitroglyoxime and lead acetate, and is said to explode when ignited. Bamberger also formed explosive hydrazine,
potassium and silver salts.
Nitroglyoxime: 5g glyoxime was dissolved into 150ml diethyl ether, and poured into a 250ml measuring cylinder. Nitrogen dioxide was produced by
reacting a piece of copper pipe with 85ml 70% nitric acid, this red gas was piped using PVC tubing into another flask to cool the gas and condense and
trap any liquid, and from that flask into the measuring cylinder holding the glyoxime solution (figure 4). The nitrogen dioxide was passed through the
solution until the reactor was exhausted. The ether solution was filtered and evaporated in a 1000ml wide form beaker in the sun until dry.
<center><img src="http://www.sciencemadness.org/scipics/axt/glyoxime-no2.jpg">
<i>Figure 4: Nitroglyoxime apparatus</i></center>
The only product obtained was a yellowish red explosive oil that smelled of nitrogen oxides and flashed when heated on a spoon.
To reclaim any nitroglyoxime that may have been present in the oil, it was poured into an aqueous solution of lead acetate this produced a red
solution and a small quantity of yellow precipitate that was filtered and dried. In small quantities the putative lead nitroglyoximate flashed when
ignited from a flame (figure 5). Glyoxime itself will not form a precipitate from lead acetate.
<center><img src="http://www.sciencemadness.org/scipics/axt/nitroglyoximate.jpg">
<i>Figure 5: Ignition of 0.1g putative lead nitroglyoximate</i></center>
The synthesis of nitroglyoxime was also attempted by the action of N2O4 on solid glyoxime, when a stream of NO2 was piped over the glyoxime in an
improvised glass condensor cooled by NH4NO3/H2O, the reaction was hypergolic, bursting into flames.
A review article by Riebsomer[6] shows reactions of nitrogen oxides with oximes, which mentions that the oxime/glyoxime groups themselves are open to
attack from NO2. For example the primary product of nitrogen dioxide acting on dimethyl, methyl, ethyl methyl and phenyl glyoxime in ether was a
glyoxime peroxide containing a R-C=N-O-O-N=C-R ring bound through the carbons. The reactions of glyoxime itself are not mentioned and no references to
the glyoxime peroxide structure could be found in recent literature.
<font size="5">Diaminoglyoxime (DAG)</font>
<img src="http://www.sciencemadness.org/scipics/axt/diaminoglyoxime.jpg">
Diaminoglyoxime also known as oxamidoxime has been prepared by numerous ways, such as from cyanogen and hydroxylamine[15], ammonolysis of
dichloroglyoxime diacetate, from dibromofuroxan and ammonia, by the reaction of dithiooxamide (rubeanic acid) with hydroxylamine[23], glyoxime with
hydroxylamine[24] but most conveniently in one step through the condensation of glyoxal and hydroxylamine[16] . DAG is soluble in hot water though
difficultly soluble in cold water and alcohol[7] so is readily precipitated, and recrystalised from aqueous solution.
Diaminoglyoxime from glyoxime: Into a beaker containing a solution of 20g (0.5mol) sodium hydroxide in 90ml water was added 17.6g (0.2mol) glyoxime.
27.8g (0.4mol) hydroxylamine hydrochloride was then added in one portion. An improvised reflux condenser was added to the beaker (figure 6) and it was
heated in an oil bath at 90°C for 6 hours. After the 6 hours the solution was cooled to room temperature which precipitated diaminoglyoxime as small
fine needles (figure 7) which were filtered and dried. Yield was 12.2g (51%)
<center><img src="http://www.sciencemadness.org/scipics/axt/reflux-app.jpg">
<i>Figure 6: Reflux apparatus for glyoxime/glyoxal to diaminoglyoxime condensation</i></center>
<center><img src="http://www.sciencemadness.org/scipics/axt/dag-glyoxime.jpg">
<i>Figure 7: Precipitate of diaminoglyoxime</i></center>
Diaminoglyoxime from glyoxal: 140g (3.5mol) sodium hydroxide was dissolved into 400ml water, the solution was cooled down to 0°C and 222g (3.2mol)
hydroxylamine hydrochloride was added in portions with stirring. To the still cold solution was added 116g (0.8mol) of 40% glyoxal in one portion. The
solution was left in the freezer for 10 minutes then placed in an oil bath heated to 90-100°C (figure 6) for 5 hours. After this time a precipitate
had formed in the solution, it was taken off the heat and cooled to 0°C. The large crystalline precipitate was filtered, placed in another beaker and
enough water was added to make up 400ml of solution. This was boiled to redissolve the DAG then on slow cooling long straw-like crystals formed in the
solution (figure 8), these were filtered and dried. Yield after recrystalisation was 38g (40%).
<center><img src="http://www.sciencemadness.org/scipics/axt/dag-crystals.jpg">
<i>Figure 8: Recrystalisation of diaminoglyoxime</i></center>
<font size="5">3,4-Diaminofurazan (DAF)</font>
<img src="http://www.sciencemadness.org/scipics/axt/diaminofurazan.jpg">
Diaminofurazan is the precursor to a wide range of energetic substances carrying the furazan ring, furazans have many desirable properties for an
energetic material such as its dense planar structure, stabilizing aromatic nature and energetic oxygen in the ring, many furazan derivatives also
have a very high heat of formation.
DAF is formed from the base catalysed dehydration and cyclisation of diaminoglyoxime by aqueous sodium[23] or preferably potassium hydroxide[24,18] at
180°C. Recently a convenient microwave mediated synthesis has also been reported[22].
DAF melts at 180°C, decomposes an 240°C and has a density of 1.61g/cm3 [25].
Diaminofurazan: 9g potassium hydroxide was dissolved into 76ml water, then poured over 24g diaminoglyoxime in a stainless steel reactor (figure 9).
The reactor was made by welding together stainless steel water pipe fittings and had a capacity of 270ml. The reactor was immersed into oil and heated
up to 180°C over 30 minutes and then left for two hours at 170-180°C, after this time the hotplate was then turned off and allowed to cool slowly.
On opening the reactor a small amount of pressure was released and the solution smelled strongly of ammonia. A quantity of small white needle like
crystals (figure 10) had precipitated which were filtered and dried. Yield was 8.6g (42%) of DAF.
<center><img src="http://www.sciencemadness.org/scipics/axt/pressurereactor.jpg">
<i>Figure 9: Stainless steel reactor</i></center>
<center><img src="http://www.sciencemadness.org/scipics/axt/daf-crystals.jpg">
<i>Figure 10: Crystals of 3,4-diaminofurazan in transmitted and reflected light</i></center>
DAF, being a weak base has been shown to form a nitrate salt, however the weakly bound nitric acid is lost when drying under vaccuum[5]. It also will
form stable complexes with some metal salts such as with copper nitrate,
Cu(DAF)<sub>2</sub>(H<sub>2</sub>O)<sub>2</sub>(NO<sub>3</sub> <sub>2</sub> [5]. <sub>2</sub> [5].
<font size="5">3,3’-Diamino-4,4’-azoxyfurazan (DAAF)</font>
<img src="http://www.sciencemadness.org/scipics/axt/diaminoazoxyfurazan.jpg">
While DAAF is yet to find commercial or military use due to limited commercial production, its explosive properties (table 1) show it to be a
practical and powerful explosive. DAAF has a relatively high density, low sensitivity to impact and a high velocity of detonation. DAAF also has
acceptable thermal stability, melting with decomposition at 249°C[14].
DAAF is formed by the oxidation of DAF by a mixture of hydrogen peroxide and sulphuric acid[9].
3,3’-Diamino-4,4’-azoxyfurazan: Into 15g 50% hydrogen peroxide was added 10g crushed ice, then 14g sulphuric acid was dripped in maintaining
temperature below 20°C. This solution was then poured over 2.5g diaminofurazan in a 80ml beaker. The suspension was stirred by use of a drill press,
whereby the DAF went into solution imparting a green colour from the formed, soluble nitroso compound. Within an hour the green colour gave way to
orange due to a fine precipitate of DAAF (figure 11). The solution was stirred for 9 hours then left for a further 15 for a total of 24 hours. The
mixture was then diluted with an equal volume of water, filtered to recover a fine orange crystals (figure 12), washed with 200ml of cold water and
dried. Yield after bottling was 1.5g (57%).
<center><img src="http://www.sciencemadness.org/scipics/axt/daaf-oxidation.jpg">
<i>Figure 11: Colour change during oxidation of DAF to DAAF</i></center>
<center><img src="http://www.sciencemadness.org/scipics/axt/daaf-crystals.jpg">
<i>Figure 12: Crystals of DAAF</i></center>
<font size="5">3,3’-Dinitro-4,4’-azoxyfurazan (DNAF)</font>
<img src="http://www.sciencemadness.org/scipics/axt/dinitroazoxyfurazan.jpg">
Further oxidation of amine groups on DAAF results in the extremely powerful explosive 3,3'-dinitroazoxyfurazan. DNAF’s explosive properties rate it
as one of the most powerful of the conventional explosives with exceedingly high velocity of detonation, very high heat of formation and good oxygen
balance. DNAF is also castable, melting at 110-112°C and boiling at 270°C[18]. However its main fault is its high sensitivity to impact, being neary
twice as sensitive as PETN, which would limit practical use. DNAF’s explosive properties are listed in table 1.
The literature methods for DAAF oxidation to DNAF requires ammonium persulphate[18,19] or sodium persulphate[8], with hydrogen peroxide and sulphuric
acid, yield was 60%. DNAF can also be formed in by direct oxidation of 3,3’-diamino-4,4’-azofurazan or DAF, but with reduced yield (15% & 4%
respectively[18]).
3,3’-Dinitro-4,4’-azoxyfurazan: Into a 250ml conical beaker was added 27.5g 50% hydrogen peroxide diluted with 17.5ml water it was then chilled to
5°C. 30g ammonium persulphate was then dissolved into the peroxide solution and left to cool in the freezer. Another solution was made by dissolving
2.5g DAAF into 32g 98% sulphuric acid and was slowly added to the cooled peroxide solution maintaining the temperature below 20°C, on addition the
DAAF precipitated as a very fine orange suspension. The beaker was then placed in an oil bath heated to 40°C and stirred by use of a drill press
(figure 13). Good stirring must be used to churn the foam that is created during the oxidation. This was maintained for 8 hours whereby the orange
solution turned bright yellow. The solution was then drowned in 200ml ice cold water and filtered, flushed with more water then dissolved into 150ml
dichloromethane, the DCM solution was then washed with dilute sodium bicarbonate/water solution. The Yellow DCM solution was then separated and
evaporated to yield yellow crystals of DNAF (figure 13). Yield was 1.3g (40%).
<center><img src="http://www.sciencemadness.org/scipics/axt/dnaf-oxi.jpg">
<i>Figure 13: Oxidation of DAAF to DNAF (left); DNAF crystals (right)</i></center>
<center><img src="http://www.sciencemadness.org/scipics/axt/dnaf-lead.jpg">
<i>Figure 14: DNAF & PETN detonated against lead block</i></center>
<center><img src="http://www.sciencemadness.org/scipics/axt/dnaf-ignite.jpg">
<i>Figure 15: Ignition of DNAF</i></center>
The DNAF was pressed into a drinking straw, taped to a lead block, buried under sand and detonated. PETN detonating cord was used for comparison
(figure 14). When touched with a match DNAF will melt then ignite and burn vigorously with a luminous smokeless flame (figure 15).
<font size="5">3-amino-3’-azido-4,4’-azoxyfurazan (AAAF)</font>
<img src="http://www.sciencemadness.org/scipics/axt/aminoazidoazoxyfurazan.jpg">
DAAF, being a basic, aromatic primary amine is open to diazotisation and subsequent reactions. The azido and diazido derivative of DAF is known to
the literature[18,21]. DAAF like DAF has only weakly basic properties thus diazotisation must be carried out in nitrosylsulphuric acid, and the formed
diazonium sulphate salt of these furazans is not stable enough to be isolated[20]. AAAF is formed by reacting a
3-amino-4,4’-azoxyfurazan-3‘-diazonium sulphate solution with sodium azide[18].
2H<sub>2</sub>SO<sub>4</sub> + NaNO<sub>2</sub> → NO.HSO<sub>4</sub> +
NaHSO<sub>4</sub> + H<sub>2</sub>O
NO.HSO<sub>4</sub> + Fz-NH<sub>2</sub> → [Fz-NH-NO] → [Fz-N≡N.OH] →
Fz-N≡N.HSO<sub>4</sub> + H<sub>2</sub>O
Fz-N≡N.HSO<sub>4</sub> + NaN<sub>3</sub> → Fz-N=N=N + NaHSO<sub>4</sub> + N<sub>2</sub>
The substitution of one amino group with an azido group greatly increases the energetic potential of DAAF with AAAF having calculated explosive
properties similar to that of HNIW (table 1). The crude un-neutralized product on drying turned from light yellow to orange, and flashed on ignition
by flame (figure 16).
3-amino-3’-azidoazoxyfurazan: 1g (15 mmol) sodium nitrite was dissolved in 28g 98% sulphuric acid and combined with a solution of 1.1g (5 mmol) DAAF
in 18g 98% sulphuric acid while keeping temperature at 0-5C. Maintaining the temperature at 0-5°C the combined solution was diluted with 26g glacial
acetic acid, then with good cooling was treated with 1g (15 mmol) sodium azide in 20g water drop by drop over 30 minutes, not letting the temperature
rise over 5°C. On addition of the sodium azide the solution foamed with release of nitrogen and turned from dark orange to light yellow. The solution
was then left for a further 15 minutes then drowned in 300ml cold water. A light yellow precipitate of AAAF was filtered, flushed with water and
dried. Yield was 0.6g.
<center><img src="http://www.sciencemadness.org/scipics/axt/aaaf-ignite.jpg">
<i>Figure 16: Ignition of AAAF</i></center>
<center><img src="http://www.sciencemadness.org/scipics/axt/explosive-props.jpg"></center>
<font size="3">References</font>
1] Pratorius-Seidler, G. “<i>Zur Kenntniss des Cyanamids</i>” Journal für praktische Chemie, 21, 129-150 (1880)
2] Urben, Peter (editor: Leslie Bretherick). "<i>Bretherick’s Handbook of Reactive Chemical Hazards"</i>. 5th ed. vol. 1, 0799.
Butterworth-Heinemann, 1999.
3] E. Bamberger & U. Suzuki, “<i>Űber Nitro-Glyoxim</i>” Berichte der Deutschen Chemischen Gesellschaft, 45, 2740-2758,
(1912)
4] Fedoroff, B. et al. “<i>Encyclopedia of Explosives and Related Items</i>“. vol. 6 pg. G119. (1974)
5] C. E. Stoner et. al., “<i>Thermal Decomposition of Energetic Materials. 48. Structures and Decomposition Mechanisms of Copper (II)
Complexes of Furazans ( 1,2,5-Oxadiazoles)</i>”. Journal of Inorganic Chemistry, 30, 360-364, 1991.
6] J. L Riebsomer, “<i>The Reactions of Nitrogen Tetroxide with Organic Compounds</i>”, Chemical Reviews, 36, 157-233, 1945.
7]Banks, C & Voter, R. “<i>Water-Soluble 1,2-Dioximes as Analytical Reagents</i>” Analytical Chemistry.; 21(11); 1320-1323 (1949)
8] Cannizzo, L. Hamilton, R. Highsmith, T. "<i>Furazan-Based Energetic Ingredients</i>". Thiokol Propulsion, Brigham City. USA. (1999)
9] Hiskey, M. et. al. "<i>Use of 3,3'-diamino-4,4'-azoxyfurazan and 3,3'-diamino-4,4’-azofurazan as insensitive high explosive
materials</i>". US patent #6358339 (2002)
10] Sheremetev, A. et al. "<i>Dinitro Trifurazans with Oxy, Azo and Azoxy Bridges</i>". PEP, 23, pg. 142-149. (1998)
11] Zelenin, A., Stevens, E. and Trudell, M. "<i>Synthesis and structure of
4-[(4-nitro-1,2,5-oxadiazol-3-YL)-NNO-azoxyl]-1,2,5-oxadiazol-3-amine</i>" Office of Naval Research, University of Illinois at Urbana-Champaign.
(1996).
12] Nielsen, A. "<i>Caged polynitramine compound</i>" US patent #5693794 (1997)
13] Dobratz, B and Crawford, P. "<i>LLNL Explosives Handbook - Properties of Chemical Explosives and Explosive Simulants</i>" Lawrence
Livermore National Laboratory. California. (1985)
14] Beal, R & Brill, T. "<i>Thermal Decomposition of Energetic Materials 77. Behavior of N-N Bridged Bifurazan Compounds on Slow and Fast
Heating</i>" PEP, 25, 241-246 (2000)
15] Barham, D. et. al. “<i>The Structure of Amidoximes. II. Oxamidoxime</i>” Journal of Organic Chemistry.; 1963; 28(1); 134-136.
16] Trudell, M & Zelenin, A. “<i>A Two Step Synthesis of Diaminofurazan and Synthesis of N-Monoarylmethyl and N,N’-Diarylmethyl
Derivatives</i>” Journal of Heterocyclic Chemistry, 34, 1057 (1997)
17] Michelman, J & Olofson, R. “<i>Furazan</i>” Journal of Organic Chemistry, 30(6), 1854-1859 (1965)
18] Boyer, J., Gunasekaran, A. & Trudell, M. "<i>Dense Energetic Compounds of C, H, N, and O Atoms. IV. Nitro and Azidofurazan
derivatives</i>" Heteroatom Chemistry. 5 [5/6], 441 (1999)
19] Beal, R. “<i>Structures and Chemistry of Amino and Nitro Furazans</i>” Thesis, University of Delaware (2000)
20] Rakitin, O. et. al. “<i>Synthesis and reactivity of furazanyl- and furoxanyldiazonium salts</i>” Russian Chemical Bulletin, 42,
No. 11, 1865-1870 (1993)
21] Tselinskii, I; Mel'nikova, S; and Vergizov, S; Zh.. Org. Khim., 17, 1123 (1981) [“<i>Azido Furazans in the Synthesis of Condensed
Systems</i>” Russian Journal of Organic Chemistry., 17 (1981) (Engl. Transl.)].
22] Kusurkar, R. et. al. “<i>Microwave mediated fast synthesis of diaminoglyoxime and 3,4-diaminofurazan: key synthons for the synthesis of
high energy density materials</i>”. Journal of Chemical Research, April, 245-247 (2005)
23] Boyer, J. and Gunasekaran, A. “<i>Dense Energetic Compounds of C, H, N, and 0 Atoms. III. 5-[4-Nitro-(1,2,5)
oxadiazolyl]-5H-[1,2,3]triazolo[4,5-c][1,2,5]oxdiazole</i>” Heteroatom Chemistry 4[5], 521-524. (1993)
24] Gunasekaran, A.; Jayachandran, T.; Boyer, J. & Trudell M. “<i>A Convenient Synthesis of Diaminoglyoxime and Diaminofurazan: Useful
Precursors for the Synthesis of High Density Energetic Materials</i>”. Journal of Heterocyclic Chemistry, 32, 1405-1407, (1995)
25] Sheremetev, A. et. al. “<i>Furazan Derivatives,: High Energetic Materials From Diaminofurazan</i>”. Proceedings of 22nd
International Pyrotechnics Seminar. Fort Collins, Colorado. 377-388. (1996)
Thanks goes to Chris the Great for sourcing 8, 11, 19 and 24. Also thanks to Chemoleo for translating 1 and 3.
[Edited on 19-5-2006 by Axt]
|
|
|
IPN
Hazard to Others
  
Posts: 156
Registered: 31-5-2003
Location: Finland
Member Is Offline
Mood: oxidized
|
|
This is just beautiful! Excellent work Axt! 
Can't wait to get to try these myself!
Would it be possible to get those references uploaded somewhere, or are they mostly paper copies?
|
|
|
Axt
National Hazard
   
Posts: 817
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Yeh, I have most as pdf's. I'll work on finding and uploading them.. they are scattered everywhere right now. I planned on uploading them, but
HTMLerising the above was frustrating enough for today. I'll get'em up soon.
|
|
|
Chris The Great
Hazard to Others
  
Posts: 463
Registered: 29-10-2004
Location: Canada
Member Is Offline
Mood: No Mood
|
|
Wow! Looks like you've beaten me to making DNAF (for those not in the know, I've done a very large amount of research into these compounds as well,
but have no glyoxime so the synth has been delayed)... that's ok though, this was the most interesting read I've had in a while. It would help to
rotate your table at the end the right way though, it's hard to read when sideways.
Great job 
Did you ever try the microwave synth of DAG again? Or try casting the DNAF? I'd really like to see what effect the cast stuff has on the lead
block... though you probably used it all up already.
Also, there are alternate methods of oxidation, I have a refference sitting right in front of me... but my scanner is not working at the moment. It
has a large amount of information on it. The ref is
Tat'yana S. Novikova et al (couldn't be bothered to type all ten names) An Effective Method for the Oxidation of Aminofurazans to
Nitrofurazans, Mendeleev Communications, 1994, pg 138-140
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
Excellent work. This will take a bit of time to prepare for the publications section as I'm working longer hours now and this is pretty lengthy.
PGP Key and corresponding e-mail address
|
|
|
Axt
National Hazard
   
Posts: 817
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
OK, zipped all journal references (all except 2,4 & 13). They are for the most part named by their title but some may be a bit off.
<a href="http://explofiles.mytoplist.net/banners/furazan-refs.zip">Download glyoxime and furazan references - 14.6Mb</a>
Table wouldn't fit unless sideways, and doesnt matter as a pdf, as you can hit rotate easy enough.
No I never did try the microwave synth again. I'm not sure what wattage mine is but it was real difficult to keep under control making me think it may
be 1200W or so, so if someone could try in 800W as specified by ref. 22 it may be a very simple way to DAF.
I never cast then detonated DNAF, only melted it to check its MP. It would be good to take a density of the cast DNAF as well but I didnt have enough
nor accurate enough scales to do that. I've no more DNAF on hand.. only about 9g of DAF left now which I might find other uses for.
Are the alternate oxidation methods easier though? cause persulphate/H2O2/H2SO4 is rather trivial. I only had (NH4)2S2O8 but Na2S2O8 is said to be
better, less foaming. Thats in one of your refs I think.
|
|
|
kazaa81
Hazard to Others
  
Posts: 368
Registered: 30-4-2004
Member Is Offline
Mood: ok
|
|
nice text Axt!
Thanks for writing this 
|
|
|
Chris The Great
Hazard to Others
  
Posts: 463
Registered: 29-10-2004
Location: Canada
Member Is Offline
Mood: No Mood
|
|
Yes, Na2S2O8 works better on scaled up batches since it reduces foaming. So, probably one of my references.
The alternate oxidation methods would probably give you a higher yield in less time. Some of the mixes just used 90% H2O2 but there was some mixes
with Na2WO4, and these had about 5 times as much active oxygen concentration. When using a much stronger oxidant mixture, the authors claimed that
you then had to cool it, the reaction went faster, and presumably gives a higher yield if you don't let it get too hot.
This might beat heating the mix and stirring for hours on end, as opposed to cooling with a salt-ive bath and stirring for 30 minutes. There was not
much experimental detail, but shouldn't be hard to adapt the procedure with the info you do have.
I am not sure if I gave you the one that was 100 some odd pages long, it had the synthesis of twenty or so of these compounds, tons of data on
crystals structure etc. It was somebodies PhD thesis, man, I would love to do that kinda work. If you don't have it I'll upload it (don't remember
the name, and haven't checked all the ones you have).
EDIT: right after I unpacked the bunch, I saw it there. It's the "Structure and Chemistry of Amino and Nitro Furazans" one in case anybody was
curious.
[Edited on 19-5-2006 by Chris The Great]
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
Take a look and tell me about typos or other problems that I may have missed:
https://www.sciencemadness.org/member_publications/energetic...
PGP Key and corresponding e-mail address
|
|
|
Axt
National Hazard
   
Posts: 817
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
For some reason, when I copied and pasted it into here it fused some words together that couldn't be edited out, you fixed some but "at180°C" and an
out of place superscript "11" is on pg. 8. "180°Cover" on pg. 10. Pages 13 & 14 need to be swapped around to keep figures in order. "(Table )"
on pg 15 is missing a "1". Theres a "?" in title of reference 7 that shouldn't be there. DAAF, DNAF & AAAF molecules are really small for some
reason?
Think I'd swap pg 9 and 10 around as well so table doesn't break up DAF synth.
Thats all I could see, thanks for that.
[Edited on 20-5-2006 by Axt]
|
|
|
Axt
National Hazard
   
Posts: 817
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
OK, hate to do this but can you add the following to the quoted paragraph, just run across it now. Certainly a furoxan seems more likely then the
peroxide. That reference has been poking around for years too, sorry I missed it.
| Quote: | Originally posted by Axt
A review article by Riebsomer[6] shows reactions of nitrogen oxides with oximes, which mentions that the oxime/glyoxime groups themselves are open to
attack from NO2. For example the primary product of nitrogen dioxide acting on dimethyl, methyl, ethyl methyl and phenyl glyoxime in ether was a
glyoxime peroxide containing a R-C=N-O-O-N=C-R ring bound through the carbons. The reactions of glyoxime itself are not mentioned and no references to
the glyoxime peroxide structure could be found in recent literature.
|
In more recent literature[26] its mentioned that the action of N<sub>2</sub>O<sub>4</sub> on dinitroglyoxime forms
dinitrofuroxan. Dinitrofuroxan is a sensitive liquid explosive that decomposes slowly at room temperature. This suggests that the explosive oil
obtained above may have been an unstable nitrofuroxan.
26] Pagoria, P. et al. "<i>A review of energetic materials synthesis</i>", Thermochimica Acta, 384, pg 187-204. (2002)
[Edited on 20-5-2006 by Axt]
|
|
|
Axt
National Hazard
   
Posts: 817
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
In the table, TNT should be C<sub>7</sub>H<sub>5</sub>N<sub>3</sub>O<sub>6</sub> not
C<sub>7</sub>H<sub>5</sub>N<sub>5</sub>O<sub>6</sub>, my fault 
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
Okay, take another look now. Edit: I don't know what it is but some 'odd' characters seemed to be inserted in the original text you posted. Curly
single and double quote marks both showed up as question marks when I copied and pasted into Lyx. I thought I'd fixed all of them but you caught an
extra one in the references.
[Edited on 5-21-2006 by Polverone]
PGP Key and corresponding e-mail address
|
|
|
Axt
National Hazard
   
Posts: 817
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
I cant see anything else. Yeh, I copied & pasted from ms works which caused some funny things to happen. Should have used notepad. I could just
keep adding things but that'd just annoy you  Like.. Like..
Just found that silver complexes of DAF are relatively insoluble, combining AgNO3 & DAF solutions produce immediate precipitate, but its deflags
not very energetically. The bromate complex was also formed by stirring AgBrO3 suspension in aqueous DAF for 30 minutes, the bromate flashes on
ignition but is unlikely to have initiating properties. The complex was not as energetic as the that of ethylenediamine, which readily detonates.
Perhaps I should have stirred it in longer or thats its Ag(DAF)2BrO3 not Ag(DAF)BrO3 that I was expecting. I'll try mixing the soluble diamine complex
with DAF, hopefully getting a precipitate.
[Edited on 22-5-2006 by Axt]
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
Okay, it's been added to the index since it seems reasonably blemish-free. If you want to write up and illustrate your further experiments I can
expand the PDF at a later time.
PGP Key and corresponding e-mail address
|
|
|
Axt
National Hazard
   
Posts: 817
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
I tried to form a peroxidic product of Diaminofurazan by condensing it with formaldehyde & H2O2. It did work, the product was quite feebly
explosive but that was expected. Structure unknown but expected to be analogous to that of urea, thus empirically C4H6N4O3. On ignition it resembled
the deflagration of nitrostarch with a large soft orange flame, leaving some black residue.
<center><img src="http://www.sciencemadness.org/scipics/axt/daf-formaldehyde-peroxide.jpg"></center>
<i>Experimental</i>: 2g (0.02mol) DAF was dissolved into 50ml water containing 1.8g (0.04mol) 70% HNO3 at 60°C. The solution was allowed
to cool to 38°C. A solution containing 2.8g (0.04mol) 50% H2O2 and 3.0g (0.04mol) 40% formaldehyde was added to the DAF solution in one portion. A
slight exotherm resulted raising temperature to 42°C and a precipitate quickly formed. The solution was left for 2 days at room temperature then
filtered to recover 2.4g of a fine white powder. 75% yield based on C4H6N4O3.
Other things tried, Ag(DAF)ClO2 was less energetic then the bromate complex. Also tried condensing DAF with glyoxal then treating the acid solution
with NaNO2 hoping to get nitroso analogue of CL-15. I just got a lot of gassing and a very small quantity of precipitate, I did it at room
temperature, should have cooled it. CL-15 is a very high performace explosive though thermally unstable, decomposing at room temp. 9600ms @ 2.0g/cm3.
Its possible the nitroso analogue wouldnt exist long either.
Attaching article on hypochlorite oxidation of DAF, difurazano[c,g]-1,2,5,6-tetrazocine would be interesting, heat of formation considerably higher
then DAAF, possibly higher density and OB is no worse. And it looks attractive 
<center><img src="http://www.sciencemadness.org/scipics/axt/difurazanotetrazocene.jpg"></center>
Its "tetramer" also mentioned in the article. Has (calc.?)properties, mp: 210°C, density: 1.8g/cm3, Hf: 1075kcal/kg, VOD: 8900ms. 22nd Int. Pyro.
Sem. pg. 377 (1996)
[Edited on 16-9-2006 by Axt]
Attachment: hypohalites as reagents for the macrocyclization of diamines of the furazan series.pdf (965kB)
This file has been downloaded 2397 times
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Have nothing constructive to add to the thread since I know nothing about explosives - just want to greet Axt for such great experimentalism, keep
rocking!

[Edited on 2-11-2006 by Sandmeyer]
|
|
|
NUKE
Harmless

Posts: 17
Registered: 21-2-2006
Location: Slovenia
Member Is Offline
Mood: Detonating with the highest order
|
|
I have one question. Can i use 30% H2O2 instead of 50% H2O2 and ice
|
|
|
Axt
National Hazard
   
Posts: 817
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by NUKE
I have one question. Can i use 30% H2O2 instead of 50% H2O2 and ice
|
If you follow the reference given (<a href="http://www.freepatentsonline.com/6358339.pdf">US6358339</a> you will see that the original patent process did use 30% H2O2, I just used ice to replicate the concentration used in
the patented procedure and prevent it boiling on addition of the H2SO4. you will see that the original patent process did use 30% H2O2, I just used ice to replicate the concentration used in
the patented procedure and prevent it boiling on addition of the H2SO4.
[Edited on 21-11-2006 by Axt]
|
|
|
Axt
National Hazard
   
Posts: 817
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
The explosive properties of difurazano[c,g]-1,2,5,6-tetrazocine as attached 4 posts up were found in Journal of Molecular Structure: THEOCHEM 765
(2006) 77–83. They gave it:
Hf: 956.61 kJ/mol
Density: 1.90 g/cm3
VOD: 8820m/s
Det. pressure: 35.61 GPa
If by some chance the azoic groups could be oxidised to azoxy groups (C4N8O4) the calculated VOD was 9760m/s @ 2.04g/cm3.
|
|
|
Mardec
Harmless

Posts: 33
Registered: 29-5-2007
Location: In a Lab
Member Is Offline
Mood: No Mood
|
|
Could Dimethylglyoxime be turned into glyoxime or some other "usefull" substance?
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
Why you haven't tried microwave synth from glyoxime? It gives 70% yield from glyoxime to DAF vs 16% yours and also way faster.
|
|
|
Axt
National Hazard
   
Posts: 817
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
I have tried the microwave synthesis, though its very difficult to control in a kitchen microwave. They mention in the article[22] that a break of
30sec is given whenever it started boiling at 800W. When placed in a kitchen microwave of unknown wattage it boils very quickly and if let rest for
30sec it will boil again within ~2sec of turning the microwave back on.
Using the pressure reactor is a more fool proof, and the yields aren't as bad as you have said, I did get 17% yield but that was from glyoxal and
lower then that reported in the literature. Whereas you gave the yield from the microwave synthesis starting with glyoxime.
If you take the best reported yields starting from glyoxal in the literature you will find 43% using microwave irradiation (glyoxal-[17,
62%]->glyoxime-[22, 70%]->DAF) and 36% using the pressure reactor (glyoxal-[16, 52%]->diaminoglyoxime-[24, 70%]->DAF).
[Edited on 7-1-2008 by Axt]
|
|
|
Axt
National Hazard
   
Posts: 817
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
More on difurazano[c,g]-1,2,5,6-tetrazocine and another use for TCCA. Though the journal volumes too old to be available online.
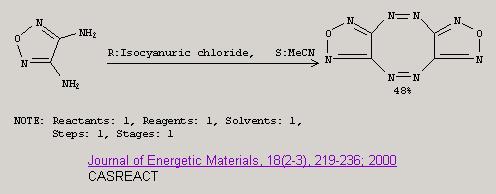
|
|
|
Axt
National Hazard
   
Posts: 817
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Heres one you may be interested in Engager, noting your post in "Announcements of Articles in Progress" thread. I believe your russian correct? a
translation of the russian patent would be good, or at least the poreparation part. I'm sure what yet, but dichloroglyoxime must have many interesting
derivatives. The needed reagents look very nice, definately beats the use of Cl2.
Yes I have been hammering scifinder of late heh.
[Edited on 24-5-2008 by Axt]
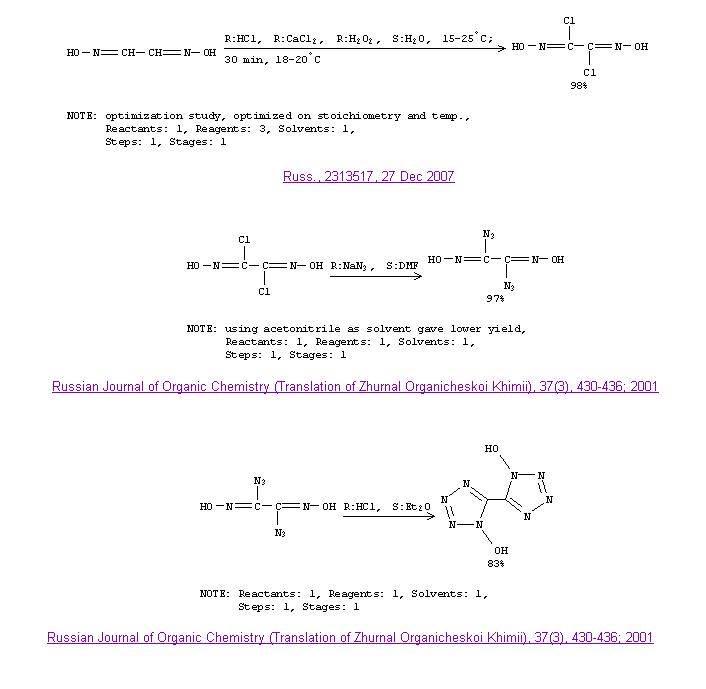
|
|
|
| Pages:
1
2 |
|