rockyit98
Hazard to Others
  
Posts: 283
Registered: 12-4-2019
Location: The Known Universe
Member Is Offline
Mood: no mood is a good mood
|
|
titanium dioxide turns yellow when heated.
store bought titanium dioxide turns yellow and back to white when cooled like ZnO do.so try to dissolve in H2SO4 to see if indeed ZnO but no such
luck. I also extracted TiO2 from old dried up white paint (not that old so Lead safe)by burning it in a kiln. they also did that .
the reason for ZnO do that is Oxygen vacancies in it when heated. maybe TiO2 have ZnO impurity.
can any one repeat this experiment with pure TiO2 ?
thanks.
"A mind is a terrible thing to lose"-Meisner
|
|
|
itsallgoodjames
Hazard to Others
  
Posts: 276
Registered: 31-8-2020
Location: America Lite
Member Is Offline
|
|
I found a paper on this with 2 seconds of research
| Quote: |
However, the TiO2 decomposes to other titanium oxides, which are TiO, Ti2O3 , and Ti3O5 , above 180°C. The titanium oxides re- main on the surface
between 200 and 330 °C. Between 330 and 400 °C, TiO is a stable oxide in comparison with Ti3O5 and TiO2
|
Nuclear physics is neat. It's a shame it's so regulated...
Now that I think about it, that's probably a good thing. Still annoying though.
|
|
|
Fulmen
International Hazard
    
Posts: 1726
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
James: That's surface oxidation of metallic Ti, not TiO2. According to Wikipedia TiO2 is reduced to TiO by metallic Ti @ 1500°C.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Johnny Cappone
Hazard to Self
 
Posts: 74
Registered: 10-12-2020
Location: Brazil
Member Is Offline
|
|
Quote: Originally posted by rockyit98  | store bought titanium dioxide turns yellow and back to white when cooled like ZnO do.so try to dissolve in H2SO4 to see if indeed ZnO but no such
luck. I also extracted TiO2 from old dried up white paint (not that old so Lead safe)by burning it in a kiln. they also did that .
the reason for ZnO do that is Oxygen vacancies in it when heated. maybe TiO2 have ZnO impurity.
can any one repeat this experiment with pure TiO2 ?
thanks. |
https://en.m.wikipedia.org/wiki/Thermochromism
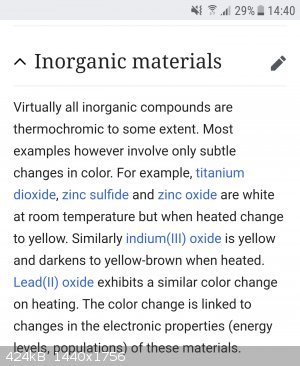
|
|
|
itsallgoodjames
Hazard to Others
  
Posts: 276
Registered: 31-8-2020
Location: America Lite
Member Is Offline
|
|
Oh. I didn't read the whole paper, as sci-hub is blocked by my school, so there was really only the title and the abstract to go on. I interpreted
the Ti metal layer to be a substrate (in the materials science sense of the word, not the chemistry sense of the word), and it wasn't actually doing
anything besides holding the titanium oxides.
Nuclear physics is neat. It's a shame it's so regulated...
Now that I think about it, that's probably a good thing. Still annoying though.
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
i believe i saw this while TIG welding titanium without shielding gas, i cant remember if it turned back to white once it cooled down, but of course
were looking at a change in structure that reports a different spectrum of light back to you when you shine white light at it, im almost sure that the
yellow remained once it was cooled down.
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
I noticed some galvanized pipe I used for an intake/exhaust would change to a yellow after running for a bit like around the 6:50 mark. I recall
buffing the snorkel to remove corrosion in the past which may have removed some zinc coating, but as the yellow seems to have formed only on one side
it momentarily caused me to wonder if some sort of dual flow was occurring during part of the phase, one side favoring a cooler intake or two columns
of air flowing past each other. In a typical pulsejet (in part of the cycle) using schlieren photography inflow at the exhaust occurs at the perimeter
while simultaneously rarified air is still exiting at the center of the tube.
Anyway I guess zinc oxide could be used for a crude thermometer.
https://youtu.be/KUYgTN1erJc
[Edited on 21-5-2021 by Morgan]
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
The TiO2 used in paint is often coated with a thin layer of SiO2 to prevent photochemical damage to the organic materials in the paint.
That can lead to some unexpected results (and slows down dissolution in acid)
[Edited on 21-5-21 by unionised]
|
|
|