RabbitTy
Harmless

Posts: 26
Registered: 19-9-2019
Member Is Offline
|
|
Method for demethylation of secondary amine?
Hi there!
Recently I have a question about the demethylation of Secondary amine. I have tried NIS(N-Iodosuccinimide, WO2017121648), didn't work out.
Doing some search, there are many ways to demethylate the tertiary amine,few for secondary amine.
appreciate any advice
structure Attached
Attachment: Structure.cdx (6kB)
This file has been downloaded 360 times
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Sorry. That file is in a format my Mac refuses to read.
|
|
|
CharlieA
National Hazard
   
Posts: 646
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
ditto
|
|
|
Dr.Bob
International Hazard
    
Posts: 2751
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
What you have presented would make a fine PhD thesis project, not a quick question here. I have done demethylations on tert amines, which already is
a big pain, and often very irreproachable but with a steroid with two secondary amines will be almost impossible to get a clean product. Good luck,
but this will not be easy.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Alkyl/benzyl chloroformates work as well as creating an n-oxide and then reacting with iron sulfate might be possibilities
|
|
|
Dr.Bob
International Hazard
    
Posts: 2751
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Do those methods work for secondary amines OK? I know ACE-chloride (alphachloroethyl chloroformate) is used for demethylating tertiary amines, but
I did not think it worked well for secondary ones. Same for the N-oxide and iron sulfate ( a trace of water is helpful). I have seen both of those
used, but the yields vary wildly, even for the same compound in the same conditions.
if you can use these or other conditions (eg, LiI in pyridine) and make it work, then congrats, as that is a tough problem. But you should plan for
some serious time and effort to make it work. And then demand a degree for it.
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
that is because it is a Chemdraw file. Here it is as a screenshot:
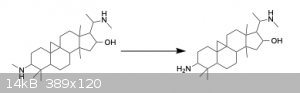
|
|
|
wxyz
Harmless

Posts: 20
Registered: 13-4-2010
Member Is Offline
Mood: No Mood
|
|
Maybe you can make the amine temporarily tertiary by adding benzyl group, then remove the group you don't like, then remove the benzyl via H2 + Pd/C.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
I realise now that I was thinking of a specific amine as a secondary amine that is actually a tertiary amine.my bad.thats all I know of n dealkylation
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
I like wxyz's idea, seems like the elegant choice. Unfortunately you've got not only 2 secondary amines but also an alcohol so targeting one amine
would be impractical.
Reflux condenser?? I barely know her!
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2799
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
What you have asked for is not "demethylate a secondary amine" -- a difficult, but possible task -- but instead, "demethylate one secondary amine and
not the other nearly identical secondary amine", which is something entirely different and almost impossible.
In any case, here is your reaction sequence:
1. Reaction with dibenzyl carbonate to the di-N-BOCylated compound.
2. Deprotonation with KH and cyclization of the upper-right NHMe and OH to a 1,3-oxazan-2-one by transesterification
3. Debenzylation of the remaining BOC group with Pd/C
That gives you a selective protection. Now:
4. N-chlorosuccinimide, base, low temperature -> N-chloroamine
5. Strong base (LDA? KH?) -> N-methyleneimine
6. Drop cold solution into warm mildly acidic water with rapid stirring -> hydrolysis
7. Further hydrolysis -> target compound
Only seven steps! Yields may vary. Not peer-reviewed. May cause unexpected death if performed wrong or at all. 
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Ummm. Maybe find an acetylating agent with a big fat ass, that will have trouble "getting to" that more sterically "impaired" amino group down South.
Use that acetylating agent, to protect your OH, and your Northern NH.
Dehydrogenate your Southern Amino function to a keto-imine. Either hydrolyse to a ketone, or displace the imine via hydroxylamine... Forming an
Oxime... Etc. Reduce to desired amine.
Remove your protecting groups.
That being said. I don't like it.
2,6-Dimethoxy-Benzoyl-Chloride? Dunno. Something bulky. Not my field of expertise.
Also of concern. Lotsa centers of asymmetry.
[Edited on 21-3-2021 by zed]
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Maybe you could kill both the secondary amines to primary amines, and use a bulky methylating agent to just remethylate get the amine up north. As a
side note, maybe there is a subtle difference in the reactions of an amine directly attached to a ring than an amine attached to a carbon chain?
Possibly exploiting that could give something.
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|