Bromolone
Harmless

Posts: 20
Registered: 16-9-2019
Member Is Offline
Mood: Aldehydic
|
|
Aesthetic Aromatics: Preliminaries
I ventured into the SciMad forum with a thread discussing feasible methods of synthesising and stabilizing o-arynes, where I realized that I needed
some more experience with laboratory procedures. After synthesising simple aromatics, I now embark on a journey (kinda similar to Tsjerk's Dimethyl
Cubane-1,4-dicarboxylate synthesis), which might seem quite lengthy or ambitious. This is a series of syntheses of compounds that I like to call
"aesthetic aromatics": mostly sym- substituted benzenes.
I've been interested in the preparation of these compounds since quite long - back when I entered the diverse realm of organic synthesis (on paper).
The motivation behind this project is simple: expanding the variety of reactions an amateur chemist can do in their lab via experimenting.
This thread focuses on the data, starter reagents, and miscellaneous stuff associated with the syntheses of the following aromatics:
1. 1,3,5-Triformylbenzene (Trimesaldehyde)
2. Benzene-1,3,5-triamine
3. Benzene-1,3,5-tricarboxylic acid (Trimesic Acid)
4. Benzene-1,3,5-triol (Phloroglucinol)
5. 1,3,5-Tribromobenzene
6. 1,3,5-Trichlorobenzene
7. 1,3,5-Trimethoxybenzene
I have deliberately omitted 1,3,5-Trinitrobenzene due to its explosive properties as a solid as well as when dissolved. As of now, I'm neither
interested in pyrotechnics nor explosives.
The first post in this thread barely provides the references from where I kickstarted. Yields from these syntheses procedures will be posted soon.
Overview
Mesitylene (1,3,5-Trimethylbenzene) is the most suitable precursor for the preparation of Trimesaldehyde for the amateur chemist (https://www.deepdyve.com/lp/springer-journal/synthesis-and-s...). Mesitylene is readily preparable by the acid-catalyzed condensation of acetone.
Benzene-1,3,5-triamine is best prepared using di-halo benzenes with an -NH2 group oriented at the m-position to both, but the reaction requires
pressure (https://patents.google.com/patent/US7217840B2/en). Trimesic acid can be easily synthesized by refluxing mesitylene with KMnO4, but the yields are
low without catalysts (https://patents.google.com/patent/CN101759558A/en and https://patents.google.com/patent/CN102146029A/en). Phloroglucinol is preparable by the acidic hydrolysis of Benzene-1,3,5-triamine. An
alternative route involves the use of resorcinol, but the method might be variably yielding (https://patents.google.com/patent/CN103641687A/en?q=phlorogl...). 1,3,5-Tribromobenzene is okay to be synthesised via the route on Orgysn (http://www.orgsyn.org/demo.aspx?prep=CV2P0592). 1,3,5-Trichlorobenzene is not obtained via the direct chlorination of benzene and requires either
the Sandmeyer reaction of 3,5-dichloroaniline or the diazotization of 2,4,6-trichloroaniline (http://nopr.niscair.res.in/bitstream/123456789/21871/1/IJCB%...).
The only (reliable) source I found for 1,3,5-trimethoxybenzene is: https://patents.google.com/patent/CN102476981A/en.
I'll soon follow this post up with troubles and issues regarding these procedures as it may be lengthened too much otherwise.
If anybody feels this to be an ambitious mistake or something alike, feel free to point out.
Comments/suggestions/corrections/improvements/additions et al are welcomed.
Thanks beforehand!
[Edited on 3-7-2020 by Bromolone]
Good documentation is key to good chemistry and software alike.
|
|
|
Fery
International Hazard
    
Posts: 1015
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
1,3,5-trinitrobenzene missing... TNT oxidation of -CH3 to -COOH using Na2Cr2O7+H2SO4 and then decarboxylation... precursor of benzene-1,3,5-triamine
you mentioned as 2.
|
|
|
Bromolone
Harmless

Posts: 20
Registered: 16-9-2019
Member Is Offline
Mood: Aldehydic
|
|
1. Trimesaldehyde
About three published papers found online focus solely on laboratory synthesis of Trimesaldehyde. One of them, which I somehow cannot relocate on the
internet, suggested the use of DABCO (1,4-Diazabicyclo[2.2.2]octane) in HCl for the oxidation of mesitylene to 1,3,5-Triformylbenzene. Given the
arduous preparation of DABCO from ethanolamine and its analogues in the presence of zeolites (https://en.wikipedia.org/wiki/DABCO), I chose to skip this method entirely.
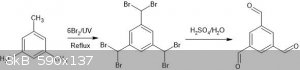
Another paper (the one referenced in the overview; https://www.deepdyve.com/lp/springer-journal/synthesis-and-s...) suggests the conversion of mesitylene to Hexabenzalbromide using bromine and UV
light and then hydrolyzing it to Trimesaldehyde in acidic medium. I, however, am not sure what will it take to separate and purify only the
Hexabenzalbromide and not lower brominated derivatives. The yields of both the intermediate steps are only about 55-60%, but the method is highly
appealing and accessible.
There exist possible alternatives to brominating mesitylene (Metacelsus in his HDDA thesis uses N-bromosuccinimide for the preparation of benzal
bromide from benzyl bromide), but there's quite a dearth of information on this on the web.
Key Barriers and Questions:
1. What reagents and conditions can be best used to di-brominate a methyl group attached to a benzene ring?
2. The Russian article preview does not show the experimental setup for preparing Trimesaldehyde.
3. A route from benzene involves the Gattermann-Koch reaction, involving toxic carbon monoxide.
4. Trimesic acid may be reduced to Trimesaldehyde, and 3,5-Diformylbenzoic acid, as well as 5-Formylbenzene-1,3-dicarboxylic acid, are expected. The
latter compound couldn't be found on the net!  The former has a boiling point of
403 °C with an error in measurement of about 40 °C! (http://www.chemspider.com/Chemical-Structure.4451440.html?ri...) Distillation at the boiling point of Trimesaldehyde (151-161 °C should not be
fairly difficult. The former has a boiling point of
403 °C with an error in measurement of about 40 °C! (http://www.chemspider.com/Chemical-Structure.4451440.html?ri...) Distillation at the boiling point of Trimesaldehyde (151-161 °C should not be
fairly difficult.
5. I was dumbstruck on reading Sigma's MSDS  : :
| Quote: |
Recommended storage temperature 2 - 8 °C. Heat sensitive.Moisture sensitive. Handle and store under inert gas. |
Are they serious?
And Fery, I had already written in the intro post that I'm skipping 1,3,5-Trinitrobenzene due to its explosive properties as a solid
and when dissolved. I've researched well into its risks in handling and storage to decide avoiding it entirely!
The reaction scheme (ChemSketch):
Good documentation is key to good chemistry and software alike.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
How about mellitic acid? And the anhydride?
|
|
|
Bromolone
Harmless

Posts: 20
Registered: 16-9-2019
Member Is Offline
Mood: Aldehydic
|
|
Mellitic acid (from this thread http://www.sciencemadness.org/talk/viewthread.php?tid=11635#... and from searching on the web) can be synthesized using hexamethylbenzene for the
moderately patient who are unwilling to use graphite as the starter compound.
A reliable method for the preparation of mellitic anhydride, accessible to well-equipped amateurs, is the heating of mellitic acid with acetic
anhydride under reduced pressure.
[I am currently working in the lab preparing the organics mentioned in the overview and didn't visit the forum for a while.]
[Edited on 9-7-2020 by Bromolone]
Good documentation is key to good chemistry and software alike.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Hexaiodobenzene, and the dication thereof (a rare sigma-aromatic compound).
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Pumukli
National Hazard
   
Posts: 705
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Perhaps you could include 1,3,5-triacetyl-benzene, synthetized from acetone and ethyl-formate. I tried this synthesis and it was definitely doable,
although the yield was not great (approx. 15% or so).
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Pumukli  | | Perhaps you could include 1,3,5-triacetyl-benzene, synthetized from acetone and ethyl-formate. I tried this synthesis and it was definitely doable,
although the yield was not great (approx. 15% or so). |
@Pumukli, do you have a reference or details of the procedure that you could post?
|
|
|
Pumukli
National Hazard
   
Posts: 705
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
I have references, yes. And I was in the process of writing a prepub article about my adventures with this synthesis when the shit hit the fan at home
culminating in a divorce. As you can see from my (in)activity on the forum I'm not really recovered yet and did not finish the mentioned article
either. I don't want to make empty promises regarding the references: they are somewhere on a back-up HDD, if I can find them fine, if not: bummer.
Anyways, the synth is fairly simple. From the top of my head it went like this: Make ethyl-formate from ethanol and formic acid. Make it dry. Make dry
acetone from standard acetone. I simply used gypsum, but mol.sieves would had been probably even better. Make sodium-methoxide (ethoxide should work
equally well) from sodium metal and dry methanol. Distill off the alcohol to get the alkoxide as dry powder. I used lithium metal beads off Ebay
instead of sodium, the making of Li-methoxide was an interesting stunt. :-) (Reaction at first needed careful thermal control, later even needed
heating up until reflux.) Make a 1:1 molar mixture of the ester and acetone in a dropping funnel and slowly let the mixture drip into the flask where
the alkoxide (some excess, mine was around 1:1:1.2 ester:acetone:methoxide) is under stirring in some dry diethyl-ether. Alcoholic alkoxide just won't
cut it! Let it drip into the flask (cc. 20 ml in say 20 minutes). Stir for a few more minutes then acidify with acetic acid. Cautiously of course...
The color of the mixture will turn yellow/faint orange (wine-like) at this point and small, golden yellow needle-like crystals should appear. Let it
sit at least overnight, the crystals will multiply! That is your product. The original reference called for an extraction step what I omited . When
tried I did not experienced any difference. Then why waste precious ether?
sym-Triacetyl-benzene is hard to dissolve in many solvents (was a bit of a surprise for me). I can't remember if I recrystalysed it at all. I took a
melting point after a solvent wash and the result was fairly close as I remember. Yes, I did not attempted a recryst, I used the crude product in my
decarbox experiments as is.
Btw. I think the process I tried to follow was from OrgSynth! Maybe a synthesis from 1935 or so, I'm not sure about this though.
Good luck with it, I'm curious about what you can crunch off from this synth as you are a dedicated organic chemist! :-)
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Thanks Pumukli, sorry to hear about your problems hope they sort themselves out!
Having prepared over 2L of ethyl formate I am now looking for things to do with it. I have already experimented with preparing formamide,
N-methylformamide, formylhydrazine etc.
|
|
|
Bromolone
Harmless

Posts: 20
Registered: 16-9-2019
Member Is Offline
Mood: Aldehydic
|
|
I'm super sorry to have stayed off the forum, as well as my lab, for this long.
COVID brought itself and some unexpected circumstances midway, and it took me a really long time to sort things out.
However, having joined university, I'm now working on research related to Schiff-base metal complexes and have resumed work on the syntheses planned
at the beginning of this thread.
Thanks a bunch, Pumukli and DraconicAcid, for your suggestions.
Good documentation is key to good chemistry and software alike.
|
|
|
|