| Pages:
1
..
43
44
45
46
47 |
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Probably, gasses formed from electrolyte reaching the LD/graphite interface produce enough pressures to crack it eventually. Funnily, never seen any
of the composite LD coated anodes crack like that...apart from the higher electrical resistance it is also likely the composite substrate anodes (or
LD itself) were probably porous enough to let most of the gas pressure escape without catastropic failure like that. Might be interesting for forming
solid LD anodes.
A thicker coat of better quality LD and pH control will probably also help...Since the part of the GSLD anode that is not submersed in the electrolyte
is hardly eroded at all at the LD/graphite interface, I wonder if you could design an almost solid lead dioxide lower (submersed) part, which is
attached to a almost complete graphite "base", which itself is not submersed, and mostly used as electrical connection. Something like that should be
possible using 2 separate LD coating runs. Maybe using this scheme, even a relatively porous LD coating would prove quite durable, even with no pH
control. Due to the very low surface area of the lower graphite part, a catastrophic failure would probably be unlikely.
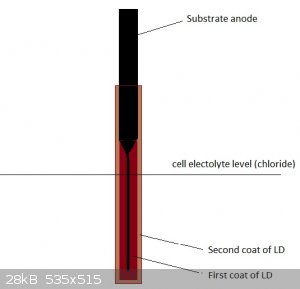
Having read about some GSLD anodes having been gone through 2 years of continuous operation before failing...wonder if the LD coating and pH control
were really good enough to protect the underlying graphite substrate for that long. A crazy thought would be that they were running an empty shell of
very porous LD, still holding on to the unsubmerged upper part of the intact graphite substrate. 
[Edited on 11-8-2020 by nitro-genes]
|
|
|
markx
National Hazard
   
Posts: 646
Registered: 7-8-2003
Location: Northern kingdom
Member Is Offline
Mood: Very Jolly
|
|
Yeah, I would kind of take such claims (2 years of continuous operation) with a slight grain of salt  All the experience collected so far suggests that such durability is rare at best, if not totally impossible on most
instances. All the experience collected so far suggests that such durability is rare at best, if not totally impossible on most
instances.
Exact science is a figment of imagination.......
|
|
|
yobbo II
National Hazard
   
Posts: 765
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
Thickness of coating was probably around 5mm of a proper coat.
They used them in the Henderson plant in Navada in the sixties.
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
I have also included a pic of my lead ion perchlorate cell here with the lead ion feeder to buildup more PbO2 by continuous leeching of Pb(ClO3)2 via
a second lead anode of smaller surface area than the immersed graphite.

|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
so I first plated PbO2 onto EDM graphite and got a uniform coating that had lots of pits due to this membrane sucking hard!!.
I notice this PbO2 was unlike my one made with lead acetate which had really bad conductivity as the thickness grew up to like 3mm when it literally
had electrical conductivity of 100 ohm or some crap.
This lead chlorate made PbO2 is highly conductive giving 0.3 ohm resistance when probed with multimeter.
This was plated at room temp with Pb(ClO3)2 and Pb(ClO4)2 solution made with membrane although this membrane isnt like my others which was properly
constructed for ion exchange electrolysis for making exotic chlorates/perchlorates, this one is for a chloro alkali cell its more permeable to the
solution and PVA would fuck off due to the strong acids involved.
anyway I added superglue to support the initial layer and it seemed to have helped stabalize the coating and after sometime the PbO2 grew around the
superglue and made a nice thick coating that doesnt peel off.

[Edited on 12-11-2020 by mysteriusbhoice]
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
made another one of these this time plated with ultrasound and very low current density of 6ma/cm^2
flaky was from previous plating attempt which sucked cuz I used too high current density.
It was extremely hard to remove the old plating that was all bumpy because even while running it in NaCl at 300ma/cm^2 it just wouldnt come off.
I had to run it as a cathode which finally took out most of it away and then plated only using ultrasonic bath with low current for this currently
nice even coating.

[Edited on 17-11-2020 by mysteriusbhoice]
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
this electrode is amazing!!!
legit its running its 2nd batch now and holding strong this time.
lead chlorate is the way to go if you dont have lead nitrate!!
although its really dangerous when let to evaporate and is prone to spontaneous decomposition when heated!!
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
one of my latest runs with it
  
old video on previous run
https://www.youtube.com/watch?v=JZWofKi_K8w
[Edited on 6-12-2020 by mysteriusbhoice]
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
new video of my perchlorate cell using PbO2-polymer composite on Ti/MMO electrodes.
the MMO died long ago due to this electrodes previous tests and failures so its prolly a Ti substrate electrode by now.
https://www.youtube.com/watch?v=ZHqiCsib0_k
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
PbO2 electrodes using the polymer composite method are easy to make just superglue + graphite/PbO2 powder onto Ti
then take this and plate in membrane plating cell with either Pb(NO3)2, Pb(ClO3)2 or Pb(ClO4)2 as electrolyte prepared from Na salts and lead anode
and do not discard the NaOH formed in the cathode compartment as it actually helps prevent migration of Pb ions to cathode side by precipitation of
insoluble Pb hydroxides on the surface in the cathode chamber.
the electrode is plated at 10 to 20 ma/cm^2 for 4-8 hours then this resulting electrode for me lasted 5 runs and 5kg of NaClO4 later XD
the 5th run I let it convert 100% and the ozone FKED IT UP hard!!
new test will be using CaSO4-graphite ceramic substrate onto Ti carrier where the ceramic is soaked in Pb perchlorate under ultrasound to soak it then
ran in the membrane plating cell so the PbO2 build up inside it before pre plating.
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
What is the caveat in this:
https://www.youtube.com/watch?v=bZMWEYiTtso
If PbO2 electrodes are so trivial to make, why are everyone using expensive Pt anodes?
I should try this as I still have the entire pack of purpose-cast lead plates hanging around. Electrolysis would be a fun side project for my boring
days.
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
cuz ppl are not chad enough to use an ion exchange electrolysis cell and a polymer/ceramic composite on Ti carrier substrate base.
I run my cell at 225ma/cm^2 and have a video of my homemade PbO2 anodes
Also heres some ammonium perchlorate 200g

|
|
|
yobbo II
National Hazard
   
Posts: 765
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
Lovly Jubbly.
A dude once stated:
Tartrate bath allows Co and Bi doping, is non smelly and easy to control. It is also convenient to prepare the bath by dissolving lead anodically. Low
lead content seems to be an advantage regarding Ti substrate, because it's easy to prepare large anodes with thin coating. No need for stockpiling
lead compounds by the 10's of kgs. Alpha seems to give way better CE too and is stress free without additives, so no peeling problems at all.
Some modifications for ATX and 12V server power supplies for constant voltage and constant current mode. Ones of the best are "HEC" brand 300W ATX
models with passive PFC. They are very easy to modify for 0-7V 0-40A use. While looking for ATX power supplies, it pays to take a look at the PCB.
Ones with (TL)494 and LM339 are almost the same design regardless the brand. Beefier examples are HP PS-3701-1(12V 60A) Dell AHF-2DC 2100W(12v 175A).
STYHI.
Yob
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
Quote: Originally posted by yobbo II  |
Lovly Jubbly.
A dude once stated:
Tartrate bath allows Co and Bi doping, is non smelly and easy to control. It is also convenient to prepare the bath by dissolving lead anodically. Low
lead content seems to be an advantage regarding Ti substrate, because it's easy to prepare large anodes with thin coating. No need for stockpiling
lead compounds by the 10's of kgs. Alpha seems to give way better CE too and is stress free without additives, so no peeling problems at all.
Some modifications for ATX and 12V server power supplies for constant voltage and constant current mode. Ones of the best are "HEC" brand 300W ATX
models with passive PFC. They are very easy to modify for 0-7V 0-40A use. While looking for ATX power supplies, it pays to take a look at the PCB.
Ones with (TL)494 and LM339 are almost the same design regardless the brand. Beefier examples are HP PS-3701-1(12V 60A) Dell AHF-2DC 2100W(12v 175A).
STYHI.
Yob |
well ive been plating out Beta PbO2 with Pb(ClO4)2 in a membrane cell where the feed is NaClO4 and lead is anodically dissolved before the electrode
to be plated is put in.
The polymer composites work great as the polymer appears to be eaten and replaced with the PbO2.
|
|
|
Jome
Hazard to Others
  
Posts: 154
Registered: 10-6-2004
Location: Soutwest sweden
Member Is Offline
Mood: desiccated
|
|
Pb/PbO2 anodes tend to have the PbO2 holding up for a while, then flaking off. I think the Pb(ClO3/4)2 bath-method seems really promising,
electrolysis is easier than distilling HNO3 to get Pb(NO3)2, which involves noxious gas-evolution and restrictable chemicals.
If I'm doing a perchlorate cell, I think the route would be a Pb/PbO2 cell to get some initial chlorates/perchlorates, then using a membrane cell to
plate PbO2 onto some kind of ideally non-restrictable substrate.
I do have a dead computer with a working PSU, but there's likely a better thread for that.
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
Quote: Originally posted by Jome  | Pb/PbO2 anodes tend to have the PbO2 holding up for a while, then flaking off. I think the Pb(ClO3/4)2 bath-method seems really promising,
electrolysis is easier than distilling HNO3 to get Pb(NO3)2, which involves noxious gas-evolution and restrictable chemicals.
If I'm doing a perchlorate cell, I think the route would be a Pb/PbO2 cell to get some initial chlorates/perchlorates, then using a membrane cell to
plate PbO2 onto some kind of ideally non-restrictable substrate.
I do have a dead computer with a working PSU, but there's likely a better thread for that.
|
another protip is that having a surfactant like benzalkonium chloride which will prevent the adherence of bubbles on the electrode you are plating.
Also if you use superglue-PbO2/graphite composite substrate as it plates PbO2 the superglue degrades due to HClO4 and is replaced slowly by PbO2 and
beware of this floaty clear/brown sludge dont light on fire!!
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
my latest Polymer-graphite substrate on Ti Beta-PbO2 plated electrode seems to work well at 207 ma/cm^2 even less errosion than the polymer-PbO2 on Ti
beta-PbO2 plated electrode.
The feed was the remainder of my dirty NaClO3 not even crystalized just boiled down contains about 10% NaCl added a bunch of sulfate additives just
now and forward feed of NaClO4 but only time will tell if it survives atleast one full conversion run past the ozone evolution stage!
The dirty chlorate feed usually causes this type of errosion to occur initially due to the chlorides.
 
Divide 27.3/17 = 16.06 amps
Surface area of one face = 77.42 cm^2
(16.06*10^3)/77.42 = 207ma/cm^2
[Edited on 20-1-2021 by mysteriusbhoice]
|
|
|
yobbo II
National Hazard
   
Posts: 765
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
For bubbles etc sticking to a substrate I wonder if you connected an old electric tooth brush to the substrate + timer that give the substrate a good
shake (vibration) for 10 seconds of every minute (say).
You can use spinning but that a bit of work making the electrical brush and setting up the spinning motor etc etc.
Surfactant is (I believe) inclined to break down and give problems.
Yob
|
|
|
RogueRose
International Hazard
    
Posts: 1596
Registered: 16-6-2014
Member Is Offline
|
|
I saw some people mentioned making lead plates as electrodes and that is something I can certainly do very easily as I have a large ingot of very old,
pure, soft lead. I also have some lead that has some antimony which is a good bit harder. Later this week, I will have some 99.95% pure: lead,
antimony, tin, bismuth, zinc and copper from which I could make an allow for the places and I can make it as thick as necessary. do you think
3/16-1/4"+ will be fine? I don't think I can make them much thinner with something in the middle like this.
So my question is whether I should try putting some wire mesh inside the plate electrode. I can use 1/4" square steel mesh or stainless mesh which I
can get in just about any size. I can also get various mesh's in aluminum.
Is there anything that needs to be done after making the plates - similar to how lead acid batteries are put in sulfuric acid to turn one side into
PbO2? Or will this be done in the process of electrolysis?
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
Quote: Originally posted by RogueRose  | I saw some people mentioned making lead plates as electrodes and that is something I can certainly do very easily as I have a large ingot of very old,
pure, soft lead. I also have some lead that has some antimony which is a good bit harder. Later this week, I will have some 99.95% pure: lead,
antimony, tin, bismuth, zinc and copper from which I could make an allow for the places and I can make it as thick as necessary. do you think
3/16-1/4"+ will be fine? I don't think I can make them much thinner with something in the middle like this.
So my question is whether I should try putting some wire mesh inside the plate electrode. I can use 1/4" square steel mesh or stainless mesh which I
can get in just about any size. I can also get various mesh's in aluminum.
Is there anything that needs to be done after making the plates - similar to how lead acid batteries are put in sulfuric acid to turn one side into
PbO2? Or will this be done in the process of electrolysis? |
The issue with surface oxidized lead as PbO2 anodes is that a perchlorate cell runs at ridiculously high current densities about 200 ma/cm^2 and that
will just shred your electrodes!!
hence why methods involving the use of a Ti substrate with a plating of conductive undercoat! like MMO or MnO2 via nitrate decomposition or cathodic
deposition of MnO2 via manganate/permanganate are used.
The salts used for PbO2 deposition I find useful are lead nitrate and lead perchlorate for conductive beta PbO2 and also lead acetate to plate some
stress free alpha PbO2 intermediate layers.
Dopants like iron and bismuth added to alpha PbO2 can even allow you to use it as an active surface layer but theres no point due to it erroding away
after a few runs.
|
|
|
RogueRose
International Hazard
    
Posts: 1596
Registered: 16-6-2014
Member Is Offline
|
|
Quote: Originally posted by mysteriusbhoice  | Quote: Originally posted by RogueRose  | I saw some people mentioned making lead plates as electrodes and that is something I can certainly do very easily as I have a large ingot of very old,
pure, soft lead. I also have some lead that has some antimony which is a good bit harder. Later this week, I will have some 99.95% pure: lead,
antimony, tin, bismuth, zinc and copper from which I could make an allow for the places and I can make it as thick as necessary. do you think
3/16-1/4"+ will be fine? I don't think I can make them much thinner with something in the middle like this.
So my question is whether I should try putting some wire mesh inside the plate electrode. I can use 1/4" square steel mesh or stainless mesh which I
can get in just about any size. I can also get various mesh's in aluminum.
Is there anything that needs to be done after making the plates - similar to how lead acid batteries are put in sulfuric acid to turn one side into
PbO2? Or will this be done in the process of electrolysis? |
The issue with surface oxidized lead as PbO2 anodes is that a perchlorate cell runs at ridiculously high current densities about 200 ma/cm^2 and that
will just shred your electrodes!!
hence why methods involving the use of a Ti substrate with a plating of conductive undercoat! like MMO or MnO2 via nitrate decomposition or cathodic
deposition of MnO2 via manganate/permanganate are used.
The salts used for PbO2 deposition I find useful are lead nitrate and lead perchlorate for conductive beta PbO2 and also lead acetate to plate some
stress free alpha PbO2 intermediate layers.
Dopants like iron and bismuth added to alpha PbO2 can even allow you to use it as an active surface layer but theres no point due to it erroding away
after a few runs. |
Thanks - I guess I'll look at doing some plating then. I am looking at ordering some Pt & Pd if the process of making MMO electrodes can be done
at home - or I'll be going with PbO2 or MnO2 I guess. I can get some titanium mesh - either diamond shaped with ~1/4 x 1/2" holes or a mesh that is
more like a sheet with lots of holes drilled in rows. IDK which would be bettr..
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
decided to also plate one of my older MMO anodes which looked like it was about to die due to being used for 8 years and the coating being highly
discolored.
the PbO2 plating on this is quite thick about 0.2mm which should be quite decent for Ti substrate anodes.
The plating is produced by first using alpha PbO2 plating bath via lead acetate in membrane cell.
Then placed in the lead perchlorate bath to plate beta PbO2 with alternating between the 2 baths in this cycle.
12 hours in the beta PbO2 bath and 30 mins in the alpha PbO2 bath where hopefully it will make this coating stable as I drive 200ma/cm^2 through it.

|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
uhh guys WTF lead substrate lead dioxide friggin works for perchlorate cells according to my latest test video on it!!
https://www.youtube.com/watch?v=p7ZbjlG_9Jk
like wow so you just have to run 12v on a big hunk of lead for 3 days in H2SO4 or Na2SO4 in a divided membrane cell to oxidize it to PbO2.
50% of it will errode but the remainder wont and it can run perchlorate cells!!
dont get your hopes up though because any future breaks in the coating and it will get fked up!!
EDiT:
yea after awhile it kinda stopped working which was to be expected.
[Edited on 1-2-2021 by mysteriusbhoice]
[Edited on 1-2-2021 by mysteriusbhoice]
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
about the superglue composite PbO2 on lead substrate PbO2 anodes they are amazing but not for perchlorates!! for making H2SO4 from CaSO4 with NaOH
catalyst they can run at 200ma/cm^2 no problem at 12v!! across the membrane and not shed during the run because heres my crop from that latest run on
that project plus a video showing the concentrated acid from the cell.
https://www.youtube.com/watch?v=t5gppFbrtWE

|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1411
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
How you anyone opinion on this electrodes. The price seems pretty good. Even free shipping. 83 Euro only. Even with M8 thread rod. Easy solid
montage.
https://www.ebay.co.uk/itm/PbO2-MMO-Anode-Cathode-electrodes...
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
| Pages:
1
..
43
44
45
46
47 |