vibbzlab
Hazard to Others
  
Posts: 241
Registered: 6-11-2019
Member Is Offline
Mood: Always curious
|
|
Vanadyl Sulfate crystals
I made some vanadyl sulfate to crystallise. It was really a task. I followed the method as per the brauers textbook of synthetic inorganic chemistry,
According to them I should get the crystals in solution ,but instead I got a really viscous blue solution which was concentrated by placing over a
waterbath. Then the remaining water was removed by placing in a DIY dessicator with anhydrous cacl2.
The compound then became very thick which was then powdered with pestle
this is the video i recorded on its prep
https://youtu.be/IQDs6neP27Y
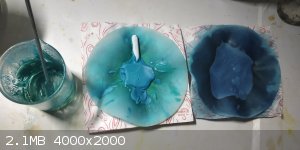
[Edited on 24-12-2020 by vibbzlab]

Amateur chemist. Doctor by profession
Have a small cute home chemistry lab.

Please do check out my lab in YouTube link below
This is my YouTube channel |
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Your material definitely contains vanadyl ions, but I do not think that you have vanadyl sulfate over here. The color is not the deep blue of vanadyl
sulfate. One test you could do is add some of the solid to water and see if it gives a clear blue solution, similar in appearance to a clear solution
of copper sulfate (a little bit more blue, deeper blue).
I have some VOSO4.3H2O, a commercial product. It looks as follows:

It is quite wet, due to its hygroscopic nature. I obtained it as shown in the picture from the chemical supplier.
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Quote: Originally posted by woelen  | Your material definitely contains vanadyl ions, but I do not think that you have vanadyl sulfate over here.
|
It may not be pure vanadyl sulfate, but the compound may have this color. I have seen many compounds that are the same, but not exactly the same
color. For example when I buy potassium dichromate it is orange, sometimes even red. I can say the same about basic copper carbonate.This is my potassium tetraperoxochromate. Same as other Wikipedias. But if you use concentated peroxide you will get a red powder that has
similar properties.
(Of course I do not mean hydrates)
|
|
|
Bedlasky
International Hazard
    
Posts: 1241
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Woelen: It may be VOSO4.xH2SO4.yH2O. I read that such a compound exist. I don't remember exact ratios VOSO4:H2SO4:H2O.
Vano.kavt: Red potassium "dichromate" is probably potassium trichromate or mixture of dichromate/trichromate. It's surely isn't pure dichromate. Look
at this.
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Quote: Originally posted by Bedlasky  |
Vano.kavt: Red potassium "dichromate" is probably potassium trichromate or mixture of dichromate/trichromate. It's surely isn't pure dichromate. Look
at this. |
I bought it a few months ago potassium dichromate and it had a reddish color while I separated these crystals from the powder and stored them
separately. You think there is no pure dichromate? I have no idea why an unopened product might have been contaminated.

|
|
|
valeg96
Hazard to Others
  
Posts: 254
Registered: 6-4-2014
Location: Italy
Member Is Offline
Mood: Moodless
|
|
Colour of many salts depends on crystal/particle size, pH and T at which it was crystallized, and other factors. Dichromate can have varying shades of
orange (the bigger, the darker) but I think, as woelen said, that vibbz's product is most likely not vanadyl sulphate.
|
|
|
Bedlasky
International Hazard
    
Posts: 1241
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
This is orange crystals, not red. At least to me  . It look like potassium
dichromate. Trichromate is really deep red. Powder have different colour than crystal. Sorry, I imagined, that your dichromate is deep red (as CrO3 or
something like that). . It look like potassium
dichromate. Trichromate is really deep red. Powder have different colour than crystal. Sorry, I imagined, that your dichromate is deep red (as CrO3 or
something like that).
https://www.amertek.co.uk/wp-content/uploads/2016/05/potassi...
This is how my K2Cr2O7 looks like.
https://upload.wikimedia.org/wikipedia/commons/6/62/Potassiu...
These crystals are bigger, so they look more redish orange.
https://en.crystalls.info/Potassium_dichromate#Gallery
And here are really big crystals, sometimes looks more redish orange, sometimes orange, depending on light.
[Edited on 25-12-2020 by Bedlasky]
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
this is my potassium dichromate. Look at someone selling on Amazon Its color is lighter than my dichromate. I mean that when I have seen the same product from different
companies the colors are often at least slightly different, as mentioned above it depends on many factors. As for the sulfate in the glass, it is wet,
see how it sticks to the glass, while the top is dry, and this also determines the color. I made nickel polyselenide yesterday. The dry looked black
in color, though I dried more and eventually remained dark gray. In short I can not judge whether this sulfate is pure, although I am not surprised
that this compound has a similar color.
[Edited on 25-12-2020 by vano.kavt]
|
|
|
vibbzlab
Hazard to Others
  
Posts: 241
Registered: 6-11-2019
Member Is Offline
Mood: Always curious
|
|
This is the solution of vanadyl sulfate that I made. I think this is it . right?

Amateur chemist. Doctor by profession
Have a small cute home chemistry lab.

Please do check out my lab in YouTube link below
This is my YouTube channel |
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
I subscribed to your channel. Now I watched the video where you make it. Pentaoxide looks pure, sulfuric acid is normal too. I think it really is
vanadyl sulfate. In general pentoxide is often contaminated. It depends on the country. They are manufactured accordingly or bought from another
country, for example a technical substance mostly are in bags, while your oxide is in a small jar, and if the stuffing is not specially or locally
sorted, it is most likely pure.
|
|
|
vibbzlab
Hazard to Others
  
Posts: 241
Registered: 6-11-2019
Member Is Offline
Mood: Always curious
|
|
thank you. Yeah the pentoxide was labgrade purchased from chemical supplies. Infact everything including acid is labgrade. Maybe as you said
contaminants maybe present insitu
Amateur chemist. Doctor by profession
Have a small cute home chemistry lab.

Please do check out my lab in YouTube link below
This is my YouTube channel |
|
|
Bedlasky
International Hazard
    
Posts: 1241
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Nice video. I have few questions:
1. Did you filter solution through glass or paper?
2. What about leftover sulfuric acid? You did recrystallization from acetone, but you evaporated it in to the dryness, so all sulfuric acid is in your
vanadyl sulfate.
Honestly, I admire you. I don't have patience for this synthesis.
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Can you try VSO4 and V2(SO4)3 ?
|
|
|
Bedlasky
International Hazard
    
Posts: 1241
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Vano: VSO4 is very air sensitive, this must be prepared under inert atmosphere. V2(SO4)3 is less air sensitive, but still it's oxidized in to VOSO4 by
oxygen (but I think that should be possible crystallize it without inert atmosphere). It would be easier to make potassium or ammonium V(III) alum,
because they are less soluble and form nice octahedral crystals.
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Quote: Originally posted by Bedlasky  | | Vano: VSO4 is very air sensitive, this must be prepared under inert atmosphere. V2(SO4)3 is less air sensitive, but still it's oxidized in to VOSO4 by
oxygen (but I think that should be possible crystallize it without inert atmosphere). It would be easier to make potassium or ammonium V(III) alum,
because they are less soluble and form nice octahedral crystals. |
Copied:
Receiving: Reduction of vanadium (V) oxide with zinc dust or reduction of vanadium (III) sulfate with zinc. Vanadium (II) sulfate, upon careful drying
of the solution, precipitates in the form of crystalline hydrate - red-violet crystals. Forms crystalline hydrates of the composition VSO 4 • n H 2
O, where n = 6, 7. Vanadium (II) sulfate heptahydrate VSO 4 • 7H 2 O is called vanadium vitriol. In air, vanadium (II) sulfate is easily oxidized by
oxygen and vanadium sulfate is produced. It also can react with cadmium sulfate and sodium fluoride and products are metalic cadmium, Na2SO4 and
Na3[VF6].
In short it is possible to make, but I do not know how much it will be possible in the home laboratory. So it should be an interesting compound for
you, because in the solution may form complexes, etc.
[Edited on 3-2-2021 by vano]
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Making VSO4 or one of its hydrates in the home lab will be VERY difficult. You need to exclude air and you need to keep temperatures low. V(2+) is
even capable of reducing water (with production of H2). Making a vanadium(III) salt is more realistic. I have experimented with vanadium(II) and
vanadium(III) in aqueous solution. Vandaium(II) slowly turns to vanadium(III) in solution, even when air is excluded. Vanadium(III) can be kept around
more easily, but is still somewhat air-sensitive. But I think that with a good desiccator and an "inert" atmosphere (here, "inert" can also be
something like propane or butane, which are OTC and cheap) you might be able to make some solid V(III) salt.
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
I know it is a bit of a specific reaction. Unfortunately, I do not have vanadium oxide now, otherwise I would definitely try.
|
|
|
Bedlasky
International Hazard
    
Posts: 1241
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Vano: I don't say that it is impossible to make VSO4 by crystallization. I say, that you need inert atmosphere.
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
I know. I wrote this information to show that simple reactions are described. Plus others will read it too.
|
|
|