metalresearcher
National Hazard
   
Posts: 757
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
How to seal a threaded stainless steel retort ?
I have a stainless steel retort made of threaded 1" pipe with a bottom welded on and a screwable top with 1" pipe thread from which a narrow pipe goes
down.
I attempted to seal the thread using ceramic wool (heat resistant and inert) but despite this, it leaks considerably, despite barely overpressure in
it (using for distilling K metal) The threaded top of the retort gets 700 C at most.
Is there a way to seal a thread gas tight ?
[Edited on 2020-10-14 by metalresearcher]
 
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
I know they silver plate SS threads to stop them sticking.
I wonder if that would also improve the seal.
|
|
|
RedDwarf
Hazard to Others
  
Posts: 166
Registered: 16-2-2019
Location: UK (North West)
Member Is Offline
Mood: Variable
|
|
Sodium Silicate?
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
tapered threads perhaps, the british swore by them forwever. I note that superheated steam lines are very thick steel, i always assumed this was for
protection, whatever but when i was thinking about this (i have no direct experience in this kind of problem btw), my first thought was that if your
male thread was of considerablely thicker gauge/wall thickness than the female, by virtue of the materials expansion under heat it would crimp or seal
in the threads better. the superheated steam parts/fittings could provided this for you.
|
|
|
Fulmen
International Hazard
    
Posts: 1716
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Exhaust assembly paste might do the trick.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Morgan
International Hazard
    
Posts: 1694
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Metal to metal seals reminded me of a pressure cooker that achieves a good seal. I bought the large one like this for sterilization of mushroom media
years ago but it's a curious question if there are any simple steel vessel methodologies that are designed to seal well at high temperatures?
"No gasket pressure cookers use a secure metal-to-metal design that is extremely tight sealing."
https://www.corriecooks.com/top-pressure-cookers-that-dont-u...
|
|
|
WolfPack
Harmless

Posts: 9
Registered: 3-2-2013
Member Is Offline
Mood: Chelating
|
|
Not sure about this, but maybe you can apply here the metal-to-metal seal concept used in a specific type of ultra high vacuum flange called ConFlat (CF).
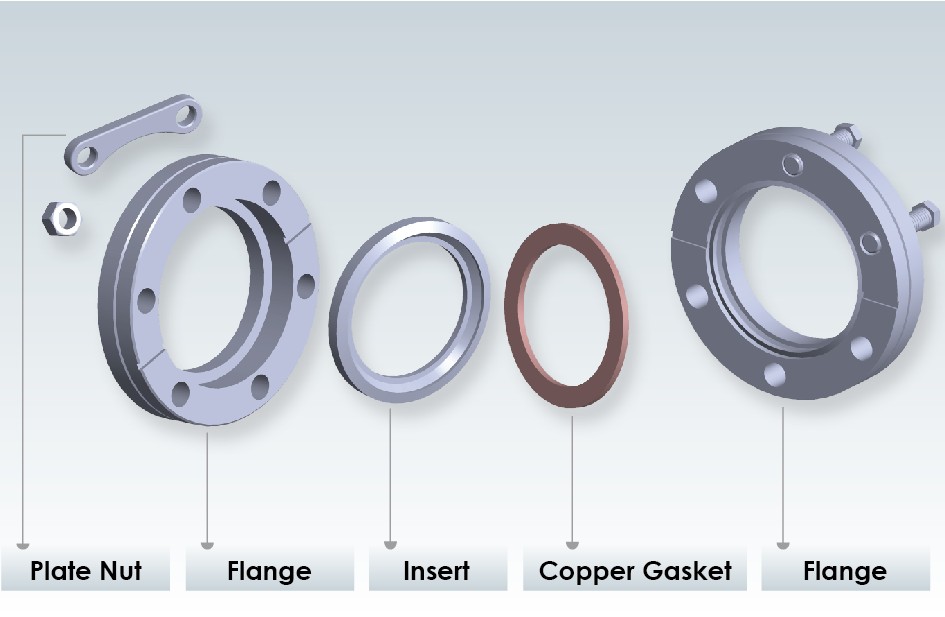
The basic idea is that you've got a flange on each side of the metal pipes that you want to join, usually made out of stainless steel, these flanges
having a series of holes for bolts. Sometimes the holes of one of the flanges is threaded (known as flanges "with tapped holes"), otherwise nuts are
required. Between them, a copper gasket is inserted. On the side that faces the other flange, they have a concentric protruding ring (called knife
edge on the image below) that embeds into the copper as you tighten the bolts, thus deforming it.

This means that you usually need to replace the copper gasket everytime you open the joint, if you want to make sure it is perfectly airtight.
Sometimes they are reused a couple of times but this is discouraged by the manufacturers - I have never taken chances anyway.
This kind of flanges is available in small sizes, which are used to connect feedthroughs for whatever process you're doing on your UHV system (e.g.
chemical vapor deposition), so maybe there is one useful to you.
Also you need to know that, especially for medium to large sizes, you need to tighten the bolts in a given pattern (have a look at this website), and even use a torque wrench, in order to uniformly apply pressure over the copper gasket.
One last thing about CF: on the first image, you might notice that, on the left side, there are both a flange and an insert. This is because the full
left-side assembly is rotatable. Once the flange is tightened, the insert can rotate. But you don't need to use these, it depends on your
system. Both of them are usually fixed-type ones.
Copper melts at 1.085 °C, so it would sustain the temperatures on your vessel. I was curious if it would form an alloy with potassium and according
to Pelton, A.D. The Cu−K (Copper-Potassium) system. Bulletin of Alloy Phase Diagrams 7, 231 (1986). https://doi.org/10.1007/BF02868995 :
| Quote: |
Because solubilities are expected to decrease proceeding from Li to Cs, the solubility of Cu in liquid K is expected to be much less than 1 at.% at
the melting point of Cu. Therefore, liquid Cu and K are assumed to be nearly completely immiscible. |
Good luck with your project! 
|
|
|
1KEE
Harmless

Posts: 25
Registered: 23-2-2020
Member Is Offline
|
|
I was thinking about copper as a gasket like that con-flat, but I'm afraid at that temperature the copper would become very soft. At room temperature,
mechanically deforming copper hardens it (work hardening) and is annealed (re-softened) at 500 to 700 deg C.
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Why not seal with teflon tape?
|
|
|
metalresearcher
National Hazard
   
Posts: 757
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
Teflon tape at 700 C ? Forget it. The copper gasket idea seems nice, but I have no flange mount, but a threaded mount.
I already tried with Na2SiO3 with water which I painted on the thread and then I closed it, filled with some wood. Wood decomposition is an ideal
leakage test as it smokes (and stinks) well. It appeared that it not leaked at the thread. I'll do another test using some stove cement to be applied
with a caulking gun (I repair my melting furnaces with it and withstands very well even till 1500 C).
And I'll test with a small amount of zinc which vaporizes at 906 C and the vapors are clearly visible. Bu a very small amount suffices.
[Edited on 2020-10-16 by metalresearcher]
|
|
|
Chemetix
Hazard to Others
  
Posts: 375
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
Carbon tape might work? A graphite impregnated silica thread wound onto the thread could also be an option.
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
Carbon powder, mixed with sodium silicate might work. It clogs the threads by two different materials that are thermally stable at those temps. Below
1000C should be rather easy still.
I've got a similar problem when I get to use my SS welded custom made pot with NPT for attempting to distill sodium metal from carbonate
carboreduction. I initially thought that little leaks may not be an issue, if they are minor and they might clog themselves with any sodium compound
that is formed during the reaction, but if not, those would be the first line I'd try out.
As a side note, when I was making CaO, I used a metal rod to stir the calcium carbonate, but I soon found out it was made of brass, because the rod
just kept going into the pot, and I quickly realized it was melting as I pushed it. It was so dirty I thought it was steel, but doh.
|
|
|
metalresearcher
National Hazard
   
Posts: 757
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
Quote: Originally posted by Fyndium  | Carbon powder, mixed with sodium silicate might work. It clogs the threads by two different materials that are thermally stable at those temps. Below
1000C should be rather easy still.
I've got a similar problem when I get to use my SS welded custom made pot with NPT for attempting to distill sodium metal from carbonate
carboreduction. I initially thought that little leaks may not be an issue, if they are minor and they might clog themselves with any sodium
compound that is formed during the reaction, but if not, those would be the first line I'd try out.
As a side note, when I was making CaO, I used a metal rod to stir the calcium carbonate, but I soon found out it was made of brass, because the rod
just kept going into the pot, and I quickly realized it was melting as I pushed it. It was so dirty I thought it was steel, but doh.
|
Na2SiO3 paste mixed with C(harcoal) powder might be a good idea. I'll try 'painting' it with a brush onto the thread.
But you attempted Na2CO3 + C ? I tried it as well as a proof of concept. I tried distilling both Na and K as I saw nice yellow resp. purplish flames,
but was not able to capture it and the retort (was still mild steel) almost melted as it requires 1200 C and the temp (propane flame) went well over
it. What kind of retort are you using ?
[Edited on 2020-10-17 by metalresearcher]
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
Wait a sec...why not simple graphite powder, locksmiths stuff.
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
It should not need over 1200C temps. I haven't yet had a chance to make that reaction due to supply shortage, and it is a good chance it might go as
far as next year.
I have thick walled stainless steel vessels for these purposes. They are welded out of min 3mm 316 steel, which takes heat pretty well. Goes bright
yellow with no issue. Haven't measured actual temps, though.
|
|
|
BromicAcid
International Hazard
    
Posts: 3245
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
I've used these types of copper anti-size / sealants before with success
https://www.jcwhitlam.com/Product/7/546/190#
|
|
|
nux vomica
Hazard to Others
  
Posts: 267
Registered: 18-7-2013
Member Is Offline
Mood: No Mood
|
|
Welding it shut , it is only way for a amateur to get a guaranteed seal , or keep threads far enough away from heat that expansion wont cause leaks ,
but you are risking the product condensing in retort by second method .
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
smear on some metal that stays liquid at 700*C, or maybe silver solder it (supposing thats possible) the top shouldnt reach 700*C nor melt the silver
otherwise some viscous grease might do the trick
as for these constructions, you should instead go for flanges, go to scrap yard and you might find some flanges, making your own flange isnt too
difficult, you just get 2 plates, weld them on sides, drill holes all the way around and then if its made perfectly it should be airtight, otherwise
some soft metal could be used to squash in between, silicone is typically used for these applications but thats lower temperature. silicone might
still work for a while, i know one guy who managed to use silicone hose for distilling WFNA- it lasted for a run or two
maybe you should just scale up the reaction chamber and weld the thing shut, should be easy to pry apart if youre going with flange design and a
cutting disc
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
700 C, is hotter than the hinges of Hell.
I had an idea, but you are going awfully hot.
I have a pressure reactor with a screw-down ring cap.
But, the cap itself, has additional bolts around its rim.
These bolts, apply pressure that is utilized to seal an inner gasket.
https://www.parrinst.com/products/non-stirred-pressure-vesse...
This isn't a bad idea. Flat metal plate, and gasket. Over end of pipe. Pipe cap, screwed down over that. Bolt protruding through pipe cap, to
produce direct pressure to sealing unit.
Pick your own gasket material.
https://www.google.com/search?q=pressure%20reactor%20diagram...
Though on reinspection, your device doesn't have much room for modifications.. Bigger?
[Edited on 28-11-2020 by zed]
|
|
|
Yo-Yo
Harmless

Posts: 26
Registered: 27-5-2012
Member Is Offline
Mood: No Mood
|
|
In the olden days mixtures called lute was used. Recipes can be found in many old chemistry texts. I've used water glass/ pipe clay mixtures. Doesn't
shrink, tolerate high temps, modest amounts of water and is mechanically stabile.
I took a PhD in neuropharmacology and all I got was this bloody t-shirt.
|
|
|
Texium
|
Thread Moved
29-11-2023 at 19:45 |