Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Metal glycinates
Rhodanide posted a photo on his Youtube channel some 3 weeks ago showing a red chromium glycinate and a blue copper glycinate salt, nice colors. I saw
glycine was readily available on eBay at a good price and ordered some.
Brauer has a report on chromium glycinate:
“CrCl3-6H2O + 3H2NCH2COOH + 3NaOH = (H2NCH2COO)3Cr + 3 NaCl + 3 H2O
An aqueous solution of one mole of green chromium chloride hydrate and 3 moles of glycine is boiled while 3 moles of NaOH is added gradually. This
gives a dark-red solution from which a violet compound separates. The latter is filtered off while the mixture is still hot. The filtrate, after
cooling and standing in vacuum over H2SO4, deposits still more of the violet compound,
together with larger red crystals. After suction-filtration and drying, the heavy red crystals are separated from the lighter violet ones by slurrying
with alcohol. In this way, both compounds are obtained in analytically pure state.
PROPERTIES:
Red crystals = chromium (III) glycinate, (H3NCH3COO)3Cr.
Violet crystals = so-called "basic" chromium (III) glycinate,
(NH3CH3COO)3Cr(OH)3Cr(OOCCH2NH3)3 • H3O”
I also came across a thesis that gave a short description of making chromium glycinate; this was different in that the sodium hydroxide was only added
after the solution turned red. Also, Poor man’s chemist synthesized chromium glycinate on his Youtube channel but he had trouble separating the
violet and red crystals. I also had various issues...
Attempt 1:
CrCl3 + 3C2H5NO2 = C6H12CrN3O6 + HCl
HCl + NaOH = NaCl + H2O
- 7g CrCl3 added to a beaker with 50ml water and stirred….note this was the anhydrous chromium salt (beautiful violet crystals).
- 11g glycine (10% excess) was dissolved in 50ml water; dissolved easily to a clear solution when hot.
- The CrCl3 did not want to dissolve, even after increasing the volume to 150ml and boiling! Reading up I saw that the anhydrous salt has low
solubility in water….
- I added the solutions together hoping that as CrCl3 is consumed more will dissolve. Heat to 80 and stir. The bit of CrCl3 that did dissolve turned
the solution black, but it was full of sparkles as the undissolved crystals swirled around. See photo.
- After 21 hrs stirring it looked very much the same, but when I dropped a few drops of the reaction solution on baking soda it bubbled showing that
acid was forming, as it should.
- After 28 hrs there was still so much undissolved CrCl3. I then dropped in 0.1g of zinc, the idea being that the zinc will reduce a tiny amount of
the chromium to chromium ii and the presence of chromium ii was said to greatly encourage the chromium iii to dissolve. That worked surprisingly well,
after only 30 minutes all CrCl3 seemed to have dissolved.
- After 50 hrs stirring hot it was still very dark, see photo. The pH was down to 2 showing it was becoming more acidic. I started adding sodium
hydroxide in intervals while checking the pH inbetween; after adding 4.8g the pH was around 7 which was almost the theoretical amount needed to
neutralize the acid that formed. It was still black, so I added a gram of glycine.
- The color started to become lighter and signs of purple! I added another gram of sodium hydroxide which pushed the pH to 8-9 and left it stirring
hot.
- At 68 hrs it was lavender-purple with suspended solids. See photo. I stopped and vacuum filtered. I got a very dark red-purple filtrate and purple /
lavender remainder.
- The remainder was dried on a steam bath, the color did not change a lot and the dry yield was 6.6g of light purple powder.
- I tried to dry the remainder on a steam bath but it remained a dark red liquid. I then put it under vacuum and over dry NaOH in the desiccator for 2
days; this gave a dark purple-red solid but it was so contaminated with sodium chloride that I chucked it out.
So at this point I only had the purple / lavender compound, which Brauer calls the basic chromium glycinate. I decided to try a different way:
Attempt 2:
Cr(OH)3 + 3C2H5NO2 = C6H12CrN3O6 + 3H2O
- 11.2g glycine was dissolved in 50ml hot water giving a clear solution
- 4.7g Cr(OH)3 was added to a beaker and the glycine solution added. The chromium iii hydroxide formed a sea-green suspension when stirred.
- At this point my stirring hot plate was occupied with something else so it was boiled a bit and then left. But it did turn grey when boiled.
- The next morning it went onto the hotplate and stirred hot for 12 hours. 2 g glycine was also added (should be 20% or so excess glycine but I do not
know if glycine comes in a hydrate or not?? The glycine I got is supposedly ‘food grade’).
- After 12 hrs the solution was only purple with no more sign of hydroxide remaining but it was left to stir hot will the next morning. See photo.
- It was vacuum filtered, and I got a purple remainder with a dark purple-red filtrate. The remainder was washed once and then onto the steam bath,
and the filtrate boiled down to under 50ml (no crystals forming).
- The remainder gave 3.7g dry purple powder, quite similar to the previous result described above, and it was added to the same vial.
- The dark filtrate was placed in the desiccator for 12 hours. This gave a hard dark purple-red layer that had to be carefully chipped out of the
crucible. See photo. I removed the flaky crystals in the centre first, they were lose from the rest and could have been excess glycinate.
- The remaining red and red-pink powder was grinded in a mortar. To a light red pinkish powder. It was then added to a beaker with 50ml ethanol, and
stirred vigorously for 10 minutes.
- When the stirring was stopped, a dark ppt quickly settled while the supernatant solution was pink with suspended solids. The pale pink supernatant
solution was decanted, and this washing with ethanol was repeated a total of 3 times. Each time the solution was slightly redder when stirred.
- After the final wash the ppt was transferred to a crucible and left in the under a steel dish to dry for 4 hours.
- Final yield was some 9.7g of reddish crystals. For sure there is still some of the purple mixed in, but I did not want to use more of my precious
99% ethanol (although it can of course be recycled).
- Final photo shows the more red and much more soluble salt on the left (the suspected normal glycinate) and the much less soluble purple salt on the
right (the suspected basic glycinate).
     
|
|
|
DraconicAcid
International Hazard
    
Posts: 4357
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Nice! (I love the sparkly purple!)
A note about how the presence of chromium(II) helps the reaction along: chromium(III), with 3 d electrons, is extremely non-labile, so ligand
exchange reactions are excruciatingly slow. Chromium(II), however, is not. A bit of zinc can reduce Cr(III) to Cr(II), the Cr(II) can then undergo
rapid ligand exchange to coordinate with the glycinate anions, and then be oxidized back to an inert Cr(gly)3 complex.
Uncatalyzed rxn: Cr(3+) + 3 gly- --> Cr(gly)3 (very slow)
Catalyzed reaction: 2 Cr(3+) + Zn --> 2 Cr(2+) + Zn(2+) (fast)
Cr(2+) + 3 gly- --> Cr(gly)3(-) (fast)
Cr(3+) + Cr(gly)3(-) --> Cr(2+) + Cr(gly)3 (fast) And then the Cr(2+) goes back to step 2.
As a general rule, any complex in which the metal is either d3, or low-spin d6, will be inert to ligand exchange. This is why so much classical
coordination chemistry was done on Cr(III) and Co(III)- the compounds were stable enough that isomers could be separated and characterized.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
DraconicAcid thanks for the explanation. We need for a few beers one day, so much to learn 
Copper glycinate shed report:
Copper glycinate was made using copper carbonate as the copper source. The expected equation:
CuCO3 + 2C2H5NO2 = C4H8CuN2O4 + CO2 + H2O.
- 15g glycine dissolved in a beaker in 75g water, with heat until a completely clear solution.
- 11g copper carbonate was added in small portions to the hot solution (near boiling) with stirring. These masses should put the glycine in slight
excess, but I do not know if either the copper carbonate or glycine is a hydrate.
- The solution immediately turned blue, and each time CuCO3 was added gas bubbles was observed.
- Stirring was continued for 30 minutes after the last addition. At this point there was no more gas bubbles.
- After stirring was stopped the solution settled into a lighter blue ppt and a dark blue supernatant liquid. See photo.
- The solution was vacuum filtered, and the lighter blue remainder washed with room temp water in the funnel. The run-through was clear on the second
wash.
- The light blue remainder was dried on a steam bath. The colour remained more or less the same, maybe got a bit lighter. Final yield of the light
blue product was 16.5g. This powder is very light and sticks to the sides of the bottle.
- The dark blue filtrate was boiled down to <50ml and then transferred to the steam bath. Beautiful dark blue crystals started to appear (see
photo).
- As the dark blue crystals dried on the steam bath, they started to turn green! See photo.
- The mix of blue and green crystals was left on a watch glass exposed to air overnight. The next morning it was mostly blue again. Yield of the dark
blue soluble product was 4.5g.
Photo of final products attached. Total weight of both products is 21g, very close to the calculated yield which was 21.5g.
So, copper also seems to give 2 products when reacted with glycine just like chromium. A ligther blue salt with very low solubility in water, and a
dark blue salt that is readily soluble. Photos online of copper glycinate usually shows the light blue product. But if one follow what Brauer says
about chromium glycinate, then the light blue insoluble copper salt may be a basic glycinate, and the dark blue salt the normal glycinate. In which
case my bottle labelling for the light blue product is wrong.
Advice from members knowledgeable with copper glycinate(s) will be greatly appreciated.
I am now busy with cobalt glycinate.
    
|
|
|
DraconicAcid
International Hazard
    
Posts: 4357
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Copper does form two glycinates (a cis and a trans), although I thought they were both slightly different shades of blue.
https://webs.wofford.edu/hilljb/Chem%20323/CopperGlycine.pdf
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Thanks for that! Doing a google image search for copper glycinate brings up all shades of blue and even green.
Do you perhaps have info on cobalt glycinate and nickel glycinate? What I see with cobalt so far is that it reacts different to copper and chromium:
it makes just a single salt, deep maroon color when wet, and quite soluble in water. There is no second salt that participates out.
From what I see online glycine can come as a monohydrate - do you perhaps know if this is the common form?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4357
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I seem to recall I once made Co(gly)3. I'd have to check my notes.
My attempt at nickel glycinate was an utter failure. Nickel readily forms a deep blue complex with glycinate, which is quite stable (almost no
precipitation of Ni(OH)2 with xs hydroxide), but I was unable to get any kind of solid from it. I'm pretty sure it's Ni(gly)3(-), but neither sodium,
potassium nor tetraethylammonium were suitable as a counterion. Adding methanol to an aqueous solution of it just gave a more dilute solution; adding
isopropanol gave me two layers.
I don't know if glycine is generally hydrated or not.
A cite for Co(II) salts of amino acids:
https://www.researchgate.net/publication/253872755_Synthesis...
Co(III) glycinate:
https://pdfs.semanticscholar.org/2c4c/4778dcf2788457fa8dc547...
[Edited on 5-8-2020 by DraconicAcid]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Nickel glycinate report:
The starting materials were nickel carbonate (which I made some time ago, see it briefly mentioned in
http://www.sciencemadness.org/talk/viewthread.php?tid=155359...
and glycine powder (food grade). The expectation was that the reaction would proceed as follows:
NiCO3 + 2C2H5NO2 + H2O = C4H8N2NiO4.2H2O + CO2
- 15g glycine dissolved in 120g water, heated and stirred into a clear solution
- Start adding nickel carbonate to the hot glycine solution. Initially a gram at a time. A reasonably vigorous reaction ensued with gas bubbling off.
And the solution turned blue. See photo. (Unlike when I did copper, with nickel the carbonate dissolved completely without forming any ppt).
- The NiCO3 was added in gram quantities and each time I waited until the foaming subsided before adding more. As the amount added increased the
reaction slowed down.
- Eventually the 10th and 11th gram took a while to react completely (some 30 and then 40 minutes) and after adding the 12th gram of NiCO3 I reduced
the heat a bit and left the solution to stir overnight. An watch glass on top of the beaker caused the steam to condense and drop back (at least when
the solution is around 50C).
- The next morning all the NiCO3 was gone but I anyway increased the heat and left it another 2 hours. I did not add any more, as 12g was the
stochiometric amount.
- I then removed the watch glass to let it boil down to just under 50ml. See photo, the color was so deep blue that on the photo it looks black.
- The solution was filtered straight into a crucible. There was no remainder on the filter paper. The crucible was placed on the steam bath (see
photo).
- When I next returned to the shed beautiful dark blue crystals were forming. See photo. The crucible was on the steam back for some 6 hours in total,
at that point the weight had stabilized for the last hour, and it was also in the ballpark of the theoretical mass. To my relief the color did not
change when drying!
- The crystals were one solid hard layer in the crucible; difficult to remove. It came lose as one piece (see photos of both sides).
- I broke it up in a mortar, just small enough to be bottled. See photos of the final product in the vial.
Mass of the recovered product was 21g, theoretical for a dihydrate was 24g. I looked for a picture of nickel glycinate online but could not find any.
I looked around with google and on Wikipedia (which has seperate pages for nickel organic salts as well as nickel inorganic salts) trying to see which
other nickel compounds are blue and while there are some it seems blue nickel compounds are not that common? Happy to be corrected if they are!
So while copper makes 2 glycinates in the same reaction with different properties, nickel (like cobalt) makes just one which is quite soluble. And
this compound also seems very stable, it did not change color when dried on the steam bath and it does not dissolve easily in concentrated HCl, unlike
copper glycinate (my go-to when cleaning the crucibles and mortar is always conc. HCl).
If any member if familiar with nickel glycinate or can find a photo I would really like to see it looks similar.
        
|
|
|
DraconicAcid
International Hazard
    
Posts: 4357
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
This is the triglycinatonickel(II) anion in solution- it's very blue. If you have six oxygen donors on nickel(II), you get a green complex; if you
have six nitrogen donors, you have a purple complex. If you have some of each in an octahedral geometry, you get blue.
I wouldn't be surprised if your glycinate isn't simply a glycinate, but a mixture of complexes.

Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
DraconicAcid - thanks mate. The color seems right I think. I'll search some more for a picture of the compound online. By the way, the bits that was
left in the crucible did eventually dissolve in the concentrated HCl, just slower than the copper and chromium glycinates / complexes.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
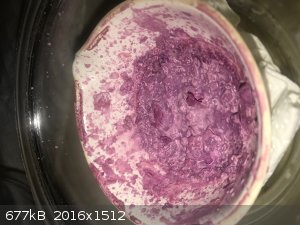
Cobalt glycinate shed report:
I actually completed cobalt before nickel but the product took days to dry in the desiccator.
I found more than 1 formula for cobalt glycinate online but the one that most often came up is C4H10CoN2O4. I then had problems getting an equation
into my stoichiometry app and had to settle for the following, which looks weird as I would not have expected oxygen.
2CoCO3 + 4C2H5NO2 = 2C4H10CoN2O4 + 2CO2 + O2
First run:
- 16.5g glycine added to a beaker with 110g water and heated to dissolve in a clear solution.
- Cobalt carbonate was added a 4g portions. The first two resulted in a obvious reaction with gas bubbling off. See photo. The reaction was much less
obvious when the 3rd portion (taking the total to 12g) was added. The beaker was left to stir overnight.
- The next morning the solution was vacuum filtered. The filtrate was very dark purple and there was a pink remainder.
- The remainder was dried on a steam bath and gave 5g of pink powder. Up to this point I assumed the remainder and the compound left in the filtrate
was two different glycinates as was the case with chromium and copper, but it did not look right. The remainder was a much lower portion than was
previously the case. I tested the dried pink remainder with hydrochloric acid and it behaved just like a carbonate! I then assumed that cobalt made
just one soluble glycinate complex and that the leftover remainder was probably excess cobalt carbonate.
- The filtrate took some 3 days to dry in a desiccator under high vacuum and over NaOH. Again, different from the copper and chromium soluble
glycinate portions. The final dry product was like a rock crust and well stuck to the crucible. See photo of the final product, purple and pink color.
Second run:
I decided to have another run trying different ratios. I took into account the apparent excess CoCO3 in run one which told me the ratio of glycinate
to cobalt carbonate should be more or less 2:1.
- 14g glycinate added to beaker with 100g water, heat near to boil.
- Add 7g CoCO3 slowly. Pink swirling solution.
- Dark solution after some 30 minutes of hot stirring.
- Add 1.8g CoCO3, pink solution (total 8.8g)
- Still pink after 30 minutes. Add 1g glycine.
- At some 45 min intervals add another 1.5g and then 1.1g glycine for total 17.6g.
- Still some hints of pink. Add 1g glycine and left stirring hot overnight.
- Next morning, a black solution. Vacuum filter hot. No remainder.
- Boil down to approx 45ml. Decided to evaporate further on steam bath to see if a different color is obtained compared to run 1 that was dried in a
desiccator. See photo of the product coming out of solution on the steam bath.
- After some total 8 hours drying on the steam bath 23g of dry pink-purple product was recovered. The appearance was quite the same as the product
from the desiccator so it does not seem to be much affected by drying at higher steam bath temperature.
Google image search brings up a picture of cobalt glycinate which is purple-ish; the color I got seems to be in the ball park.
EDIT: I found a paper "Simultaneous Preparation of Facial and Meridional Isomer of Cobalt-Amino acid Complexes and their Characterization" (that I
will try to link) that gives the following formula for a cobalt glycinate complex: C6H12CoN3O6
If I then consider the following equation:
O2 + 12 C2H5NO2 + 4 CoCO3 = 4 C6H12CoN3O6 + 4 CO2 + 6 H2O
the mass of glycine and cobalt carbonate is 10:5.2 so almost exactly what I suspected after the first run. It also says the color is pink or purple
depending on hydration, and thus how it was dried. They use a different method to make the compound, also involving a carbonate route, but much more
complex.
Attachment: Cobalt-Amino acid Complexes.pdf (160kB)
This file has been downloaded 435 times
[Edited on 7-8-2020 by Lion850]
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
I have really been enjoying this thread, thank you very much!
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Hi B(a)P thank you! Even though there may not be a great interest in glycinates I think it is good to record what I did and got in case someone looks
for info one day. Seems a reasonably straightforward way for the home amateur to get interesting coloured compounds. My favourite so far is probably
the blue nickel salt. I also need to revisit chromium to see how to get the red soluble salt more pure.
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
This one is probably more for energetic materials here is a video on copper glycinate chlorate double salt
https://youtu.be/pc1LXcweW8Y
|
|
|
valeg96
Hazard to Others
  
Posts: 254
Registered: 6-4-2014
Location: Italy
Member Is Offline
Mood: Moodless
|
|
Lion850, if you like the blue Ni salts I suggest you these other syntheses. They start from the simple glycinate and make other salts.
https://doi.org/10.1135/cccc19880563
https://doi.org/10.1021/ja01219a004
https://doi.org/10.1021/ja01606a078
Many other syntheses with other metals are:
https://doi.org/10.1002/zaac.201700217
https://doi.org/10.1590/S0100-40422002000500004
https://doi.org/10.1042/bj0140574
https://doi.org/10.1080/00958970802283065
Also, for some reason my trans- copper glycinate turned olive green after a couple of days.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Hi valeg96 thanks for the links. I don't have access to most, but I will try the manganese glycinate so etime.
|
|
|
KoiosPhoebus
Hazard to Self
 
Posts: 53
Registered: 23-1-2023
Member Is Offline
|
|
Attempt at preparing manganese (II) bisglycinate
I thought I'd just jot down a bunch of my notes on my attempts to make
Mn(Gly)2 thus far. I'm skeptical that I've gotten anything even resembling the pure compound thus far; but I thought I'd list out what I've
tried so others know that it hasn't worked, or maybe someone could suggest new ideas?
Attempt I/III: Reaction between manganese carbonate and glycine
My first attempt was to prepare manganese carbonate, and then react that directly with glycine.
To do this, I first tried adding 17.79 g of 95% manganese sulphate monohydrate to 16.80 g of sodium bicarbonate:
MnSO4 + 2 NaHCO3 → MnCO3 + Na2SO4 + CO2 + H2O
I then filtered out the precipitate, dried it and added 13.30 g (to account for the possibility that I prepared the monohydrate) to a solution
containing 15.24 g of glycine. In theory, the reaction that should have happened was:
MnCO3 + 2 NH2CH2COOH → Mn(NH2CH2COO)2 + CO2 + H2O
In practice, the "manganese carbonate" gave off a couple of bubbles and proceeded to just...sit there. After doing some reading and running some
trials with my manganese sulphate, I suspect that the monohydrate hadn't fully dissolved when preparing the manganese carbonate, and some may have
ended up in the "manganese carbonate" precipitate
So in my third run (after the attempt below), I added the manganese sulphate first, and then added 0.1 mL of concentrated sulphuric acid, allowing it
to all dissolve and form a clear pink liquid before adding in any sodium bicarbonate. The whitish precipitate was then filtered out and dried, and I
added 11.49 g of my manganese carbonate into 200 mL of water, containing 15.24 g of glycine. I wasn't entirely sure if I'd prepared anhydrous
manganese carbonate or the monohydrate, so I decided to add a smaller amount first and I could add the difference later if need be.
Unfortunately, as before, only a few bubbles were evolved. Despite leaving the mixture at 70 to 80 deg C for several hours, it remained an opaque,
pink-white mixture of mostly unreacted manganese carbonate.

I checked that the insoluble particles were mostly manganese carbonate (and not an insoluble basic glycinate or similar) by adding 1.94 g of citric
acid, which immediately produced a bunch of fizzing. More bubbles showed up upon another three additions of 1.94 g citric acid; I didn't think there
was much purpose to adding more citric acid than that.
The filtrate from the glycine/citric acid mix turned an orange-red shade; I'm unsure how much of this is colour change due to complex formation versus
colour change due to oxidation of Mn(II) to Mn(III) (something similar happened with the other method, see below).

Interestingly, with the glycine/citric acid filtrate, heating caused the colour to revert to a lighter pink more characteristic of Mn(II):

Glycine doesn't appear to be a sufficiently strong acid to decompose significant quantities of manganese carbonate, so I'm not sure if it's viable to
prepare manganese bisglycinate using this method. I'm considering using a significant excess of glycine (e.g. a 3:1 glycine:manganese molar ratio) and
then washing off any remainder using an organic solvent e.g. pyridine or formamide.
Glycine complexes seem to be very insoluble in organic solvents, so this should work; the problem is that glycine is itself not very soluble in
organic solvents so I suspect it'll require way too much solvent to get out all of the excess glycine, never mind the possibility of some weird
trisglycinate complex forming which would make removing excess glycine practically impossible.
Attempt II: double displacement between calcium bisglycinate and manganese sulphate
The method is described here: https://patents.google.com/patent/WO2002030948A2/en
Basically, the bisglycinate of calcium is prepared first by reacting calcium hydroxide with glycine:
Ca(OH)2 + 2 NH2CH2COOH → Ca(NH2CH2COO)2 + 2 H2O
Then, while the calcium bisglycinate remains in solution, an equimolar amount of manganese sulphate is added. The stability constant of the
calcium-glycine complex is quite low (log K < 1.4, data from the old NIST database of metal complex stability constants), so a relatively large
amount of calcium and glycine are uncomplexed at equilibrium. This allows the calcium to react with the sulphate anions introduced and precipitate as
calcium sulphate, while the manganese is complexed by the free glycine (the log K for Mn(Gly)2 is higher at < 4.8, though that would
still be considered a weak chelate), leading to the following equation:
Ca(NH2CH2COO)2 + MnSO4 → CaSO4 + Mn(NH2CH2COO)2
I prepared the calcium bisglycinate mixture first by adding 7.41 g of calcium hydroxide into 100 mL of water containing 15.24 g dissolved glycine. I
left it to react for about an hour at 60 - 70 deg C with constant stirring. When I returned, quite a bit of calcium bisglycinate appeared to have
precipitated from the solution, as there was a lot of fluffy, translucent particles, as opposed to the solid-white powder that the calcium hydroxide
would've appeared as. Apparently calcium bisglycinate has very low solubility (https://dpl-us.com/wp-content/uploads/2022/04/DPL-Mineral-Bi...).
I then dissolved 17.79 g of manganese sulphate monohydrate in 100 g of water, and added it into the calcium bisglycinate mixture with stirring. A
white precipitate rapidly formed, and the colour of the solution changed over time from light pink to a dark red. I was a little unsure if this was
due to complex formation or oxidation of Mn(II) to Mn(III), so I took a small sample and pipetted it into a solution of NaOH. It immediately formed a
white precipitate with at most tiny hints of brown, suggesting to me that most of the aqueous Mn remained reduced.
After leaving it to react for an hour or so, I then filtered out the precipitate with coffee filters and gravity filtration. At this point, I honestly
thought I had my manganese bisglycinate, however...

...it turned out to be impossible to dry. Despite placing it on a hotplate at max setting for a few hours, the result was a gooey sludge which handled
like slightly-melted rock candy.

Placing the dish into a bag with calcium chloride didn't do anything (if anything, the manganese bisglycinate seemed to have absorbed water?). After
various attempts at drying the solution, I gave up and tossed it out. I then repeated the synthesis through double displacement, but evaporated the
solution until enough of it remained that everything remained dissolved. I then added ethanol in an attempt to precipitate the Mn-glycine complex;
this resulted in a milky ethanol layer and another gooey orange-brown layer. Frustratingly, the ethanol layer was practically impossible to filter
dry; the small particles of precipitate simply clogged the filter.
It does seem like difficulty in drying bisglycinates isn't entirely uncommon; this post on ResearchGate: https://www.researchgate.net/post/How_can_I_synthesize_pure_... used a similar method with barium hydroxide instead of calcium hydroxide and
appears to have run into the same issue with Fe(II)-Gly. So those are the methods I've tried thus far; I'd be interested to hear from anyone else who
tried to prepare manganese bisglycinate or who might have any ideas on ways to prepare it.
|
|
|
KoiosPhoebus
Hazard to Self
 
Posts: 53
Registered: 23-1-2023
Member Is Offline
|
|
I thought I'd also describe some of the methods I have considered or am
considering for preparing more manganese bisglycinate:
Adding glycine to MnSO4
That would be from this paper: https://doi.org/10.1007/s00344-012-9259-7. The authors add an amino acid (glycine, arginine, or histidine) to FeSO4 in a 2:1 ratio,
then evaporate the solution and wash with ethanol + diethyl ether.
I have actually tried this method with FeSO4 in an attempt to prepare ferrous bisglycinate (though I only washed with ethanol - I don't
have diethyl ether unfortunately). The problem is that even after drying the tan brown final product (to the point where a small amount of it burns),
I got a yield which was 200% that of theoretical Fe(Gly)2, and which was much closer to that of the mass of FeSO4 + glycine I
added in. I did some more research and found that there was a compound known as ferrous glycine sulphate (Fe(GlyH)2SO4), where
the glycine remains protonated and the sulphate acts as a bridging ion between complexes of Fe(GlyH)2 (https://doi.org/10.1002/zaac.201500776).
The problem is that ferrous glycine sulphate doesn't really act like a "true" chelate; the protonation of the glycine means that binding to the
Fe2+ ion is monodentate as only the NH2 group coordinates with the cation. This has various assorted effects such as the higher
rate of side effects when ferrous glycine sulphate is taken in place of ferrous bisglycinate (http://dx.doi.org/10.1080/14767058.2018.1482871). In this sense, ferrous glycine sulphate behaves more like ferrous sulphate; Fe(Gly)2
is primarily taken as a supplement for its low rate of side effects vs FeSO4 and hence higher side effect rates means it's not behaving
like fully chelated iron. It's also...not ferrous bisglycinate, which was what I was trying to make.
I do wonder if the additional diethyl ether wash would convert it to ferrous bisglycinate but I'm skeptical. I've been reading quite a bit of
literature, and I don't see anything on diethyl ether being able to remove sulphate from complexes. Additionally, if diethyl ether could truly perform
this role, I doubt there'd be this many papers/patents on various methods to prepare Fe(Gly)2.
Synthesising manganese-glycine adducts from MnCl2
This is from the paper provided by a fellow Sciencemadness user above (https://doi.org/10.1590/S0100-40422002000500004). I've read it and I'm reasonably confident that this has a similar issue to the method described
above, in that the prepared compound is Mn(GlyHCl)2 and may have monodentate binding to Mn2+ instead of bidentate chelation.
If no other method works, I might try this with either MnCl2 or Mn(NO3)2, and then challenge the resulting complex
with KH2PO4 and then NaOH to see how strong the chelation effect is. Manganese citrate has a log K (measurement of how strong a
chelate is, log10 of e.g. [MnCitrate]/[Mn][Citrate] at equilibrium) of 4.2 versus manganese bisglycinate's log K of 4.8, so if I go down
this route I'll use MnCitrate as a comparison.
Double displacement between MnCl2 and lithium glycinate in organic solvent
This is from a 1985 paper where the authors prepared a whole bunch of ferrous amino acid complexes (https://doi.org/10.1016/S0020-1693(00)82257-4), mostly through some variation of preparing the lithium salt of the amino acid in methanol and then
adding the result into solution of methanol containing ferrous chloride. The ferrous complexes are typically minimally soluble in organic solvents and
hence precipitate, while LiCl is reasonably soluble in ethanol/highly soluble in methanol so it remains in solution.
To me this seems like it has the best shot at success. Unfortunately, I don't have methanol or lithium hydroxide at the moment. I'm working on
preparing the latter from ceramics-grade lithium carbonate, but I'm cautious of using methanol simply due to the potential for toxicity from fumes.
Has anyone worked with methanol before and would know whether it can be safely worked with in an indoor setting?
Personally, I'm hoping that I can get one of the double displacement methods working as I was planning on making metal complexes of other amino acids.
In particular I was looking into making histidine complexes; however histidine is a basic amino acid and hence is positively charged at neutral pH.
This makes it harder to prepare histidine complexes as its basicity means it can't be reacted with carbonates like glycine. Additionally, it has to be
introduced into an alkaline environment to be deprotonated and form a charge-neutral complex with the relevant metal. This isn't an issue in the
double-displacement methods as Ca(OH)2 or LiOH would raise the pH sufficiently to deprotonate histidine.
|
|
|
|