John paul III
Hazard to Others
  
Posts: 110
Registered: 28-4-2018
Member Is Offline
Mood: No Mood
|
|
shock sensitive mixtures of KNO3
Are there mixtures of KNO3 without additional oxidizers that can be easily ignited by shock, like by a firing pin?
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
White phosphorus is said to form an impact sensitive mixture with potassium nitrate, but that is very impractical.
I don't know for sure, but possibly red phosphorus, antimony sulfide or magnesium powder?
I am just guessing, any reason you want to know?
|
|
|
yobbo II
National Hazard
   
Posts: 765
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
I once read in a book by Seymour Leck (if I have the spelin right) that a mixture of finely divided lead + KNO3 made a detonatable mixture. Believe
it if you like. Seymour probably was not the most respected authority.
Yob
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Quote: Originally posted by yobbo II  |
I once read in a book by Seymour Leck (if I have the spelin right) that a mixture of finely divided lead + KNO3 made a detonatable mixture. Believe
it if you like. Seymour probably was not the most respected authority.
Yob |
You have got me curious to experiment with this, I will report back with what I discover.
|
|
|
Microtek
National Hazard
   
Posts: 872
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
KNO3 and red phosphorus is friction sensitive enough to be set off by firm action of ceramic pestle on filter paper, so I would think it could also be
initiated by impact.
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
IIRC 'yellow powder', being a mixture of potassium nitrate, potassium carbonate and sulfur molten together, is reported to be shock sensitive. Never
tried this myself though, and it seems pretty dangerous to prepare, because you really need to melt the components together (just mixing the dry
powders won't work), and it can detonate pretty violently in the molten state.
[Edited on 10-12-2020 by phlogiston]
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
Fulmen
International Hazard
    
Posts: 1726
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
IIRC the "secret" to yellow powder is the formation of polysulfides, so it might be possible to get some of the performance by first melting the
sulfur and carbonate, then grind down and mix with the nitrate.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
wg48temp9
National Hazard
   
Posts: 786
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
I was surprised that potassium carbonate would react with sulphur. Apparently it does in water or alcohol so I guess it would when melted together.
Attachment: potash-sulphur-davis1927.pdf (211kB)
This file has been downloaded 459 times
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
What is going on here in that like yellow powder, it seems to have an intermediate step before exploding and not just igniting or going off instantly
with high heat?
https://youtube.com/watch?v=VzyWsdWfGUY
|
|
|
Fulmen
International Hazard
    
Posts: 1726
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
wg48: It most certainly does, I've done it on several occasions to prepare polysulfides for staining silver. I believe NaOH can be used as well.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
John paul III
Hazard to Others
  
Posts: 110
Registered: 28-4-2018
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by phlogiston  | IIRC 'yellow powder', being a mixture of potassium nitrate, potassium carbonate and sulfur molten together, is reported to be shock sensitive. Never
tried this myself though, and it seems pretty dangerous to prepare, because you really need to melt the components together (just mixing the dry
powders won't work), and it can detonate pretty violently in the molten state.
[Edited on 10-12-2020 by phlogiston] |
Damn that's amazing if it works, and i will try making one gram of this
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
It is easy to find videos of the molten mixture detonating, so it definitely seems to 'work', eg:
https://www.youtube.com/watch?v=ZrYgRf-NuHs
but it'd be very interesting to see if you can melt it and cool it down before it has a chance to explode on you. There is not a lot of literature on
the properties of this material after cooling/solidifying.
[Edited on 11-12-2020 by phlogiston]
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
Σldritch
Hazard to Others
  
Posts: 310
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
I tried making nitrites that way because i read the polysulfides reduce the nitrate. I never got any though because it keept exploding even when far
from optimal mixture so kt certainly works. 
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
I wonder if a low melting solid or boric acid which is know to stabilize sulfur and nitrate mixtures would stabilize the mixture of yellow powder or
urea and nitrite mix
I'd imagine ammonium acetate and potassium nitrite would also be dangerous due to ammonium nitrite intermediate formed.
[Edited on 11-12-2020 by symboom]
[Edited on 11-12-2020 by symboom]
|
|
|
Pyro_cat
Hazard to Others
  
Posts: 243
Registered: 30-4-2018
Member Is Offline
Mood: No Mood
|
|
My potassium chlorate from bleach mixed with sugar went off with a hammer shock.
|
|
|
DBX Labs
Hazard to Self
 
Posts: 57
Registered: 24-12-2020
Member Is Offline
|
|
Haven’t seen this discussed much on other threads: Nitrite salts and Urea
Did a video on this a while back, the mix goes off on constant heating very similar to yellow powder. I melt a gram or two to the point where it was
about to detonate (you can hear it start to ramp up in decomposition right before) and broke it apart when it cooled. 5 milligrams went off with a
hammer strike on the anvil. Similar sensitivity to ETN.
|
|
|
DBX Labs
Hazard to Self
 
Posts: 57
Registered: 24-12-2020
Member Is Offline
|
|
I read a while back somewhere that a mix of KNO3, Mg, and Guanidine Nitrate form a shock sensitive mix. I’d have to assume that the paper meant more
so than the sensitivities of KNO3/Mg flash or Guanidine Nitrate on their own.
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Maybe if urea and nitrite were fine powders to start and intimately mixed and very slowly melted a slightly more uniform pre-reaction/idealized
formation of the explosive would come about, walking it up rather than racing to the finish line? It's just a thought if there is a fragileness or
more favorable condition.
Out of curiosity, does the typical yellow powder mix tolerate substituting nitrite for nitrate?
|
|
|
Nitrosio
Hazard to Self
 
Posts: 57
Registered: 31-3-2018
Member Is Offline
Mood: No Mood
|
|
KNO3 + K2S
KNO3 + P4S3
KNO3 + P + CS2
|
|
|
metalresearcher
National Hazard
   
Posts: 758
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
KNO3 is less shock semsitive than KClO3, the latter forms much more shock sensitive mixtures. Hence mixing with sulfur is not rcommended and for that
reason black powder contains KNO3 rather than KClO3.
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 441
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
"Easily"? I'm not aware of any, personally. BKNO3 is widely used in missiles as both grain ignitors and in det trains. Normally it is initiated by
high voltage dielectric breakdown by purposeful failure of a dielectric material which then bumps up amps. Usually HV with caps, piezos or flux
generators.
I'd imagine additives could make it firing pin sensitive. If you incorporated fluoropolymers and Mg, i think you might get something impact sensitive,
possibly.
THV/Al or Mg comps are sensitive to photo initiation by a nikon camera flash and sensitive to detonation by impact if heat cured in pressed form
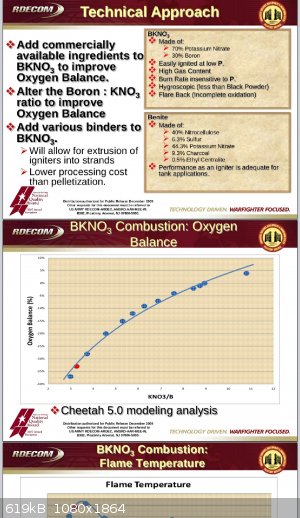 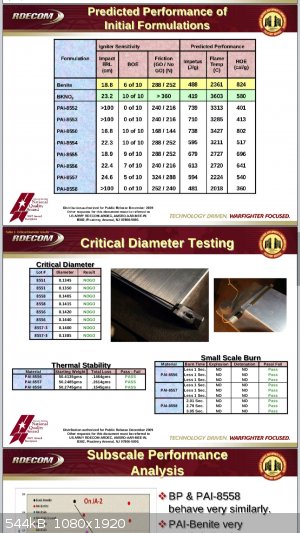
|
|
|