Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Is there a limit to what alcohols you can make with the grignard reaction? steric hinderance?
i have yet to actually do it. i'm still collecting needed supplies, but the grignard reaction fascinates me greatly because it's so versatile and
there are so many alcohols you can make with it.
is there a limit on what alcohols you can make, due to steric hinderance? what if you tried adding diethyl carbonate to tert-butyl magnesium bromide?
or tritylmagnesium bromide? or converted the product tertiary alcohols to alkyl bromides and grignard reagents and did this AGAIN, thereby creating
exponentially more massive alcohols? the final products would be very bulky and might not even be able to exist?
just an interesting thought, i can't find any literature on this issue.
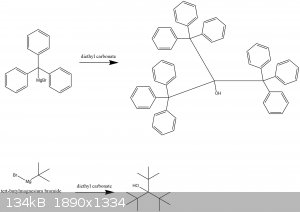
edit: the tri-tertbutyl carbinol compound does exist and has been characterized,
[Edited on 11-30-2020 by Cou]
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
The short answer is stearic hinderance will prevent the formation of increasingly bulky alcohols.
The stearic hinderance is going to make this a hard synthesis with low yield regardless of method.
But instead of diethyl carbinol, you need hexamethyl acetone.
The ethyl ether groups in diethyl carbinol are not going to replace due to stearic hinderance.
You need a better leaving group. Or in the case of hexamethyl acetone, simply adding to a ketone.
Here is the literature reference of tri-tertbutyl carbinol.
And the structure corresponds to the second structure. The yield of tri-tert-butyl carbinol is 5%
https://pubs.acs.org/doi/pdfplus/10.1021/ja01217a049
The first structure would require hexaphenyl acetone using the same method which to my knowledge doesn't exist.
I am not certain if it can be made due to the stearic hinderance.
While the compounds may be interesting, I cannot think of a practical application.
This is of course science and practical applications are not the goal.
Commercial research labs generally have a goal.
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
That is nuts, you can't even make tri-t-butyl carbinol from t-butyl lithium and hexamethylacetone.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2748
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Even if you can make a super steric Grignard, it likely won't react. Some sterically hindered Grignards are used to deprotonate other compounds,
much like butyl lithium, as they are just not very nucleophilic. I think isopropyl Grignard can even be hindered enough to do this. I'm sure there
are some reactive ketones that it will react with, but with many compounds it just does little or nothing. I'm sure that people have already
published some lists of what does and does not work, but it might be decades old.
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
I guess I have to get into organolithiums if I want to make diiisopropyl carbinol.
organic chemistry seems fun on paper, but often turns out to be disappointingly messy in reality.
[Edited on 12-1-2020 by Cou]
|
|
|
Texium
Administrator
       
Posts: 4618
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by Cou  | I guess I have to get into organolithiums if I want to make diiisopropyl carbinol.
organic chemistry seems fun on paper, but often turns out to be disappointingly messy in reality. |
You’re
finally getting it! Don’t be discouraged though. The challenges just make it more fun.
|
|
|