| Pages:
1
..
52
53
54
55
56
..
60 |
HydrogenSulphate
Harmless

Posts: 38
Registered: 13-10-2019
Member Is Offline
Mood: Caffeinated
|
|
Ferrous sulphate heptahydrate

|
|
|
HydrogenSulphate
Harmless

Posts: 38
Registered: 13-10-2019
Member Is Offline
Mood: Caffeinated
|
|
Some dyes, primarily for microscopy. Indigo carmine and fluorescein.

|
|
|
MidLifeChemist
Hazard to Others
  
Posts: 192
Registered: 4-7-2019
Location: West Coast USA
Member Is Offline
Mood: precipitatory
|
|
User name checks out
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
500 g bromobenzene
500 mL diethyl carbonate
Hoping I can do some fun grignard reactions at home. Phenylmagnesium bromide is the easiest grignard reagent to make. Not carcinogenic like alkyl
halides, commonly prepared in undergrad teaching labs.
[Edited on 11-7-2020 by Cou]
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
Finally restocked some 98% sulfuric acid.
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Quote: Originally posted by Cou  | 500 g bromobenzene
500 mL diethyl carbonate
Hoping I can do some fun grignard reactions at home. Phenylmagnesium bromide is the easiest grignard reagent to make. Not carcinogenic like alkyl
halides, commonly prepared in undergrad teaching labs.
[Edited on 11-7-2020 by Cou] |
diethyl carbonate is a fun reagent because it can be used to make tertiary alcohols from only a grignard reagent.
the alcohol is a tertiary alcohol with the alkyl group copied 3 times.
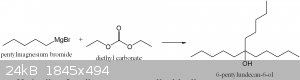
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Nice, what are you planning to do with the triphenylmethanol?
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
i think triphenylmethanol is a cool molecule itself so i just wanna make it for the hell of it.
I also want to try making esters of it, such as trityl acetate. I think you can do fischer esterification because triphenylmethanol doesn't dehydrate.
|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Some things are just destined to bring a smile.

|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
That's a really nice one! Sad that this is not possible anymore in the EU 
|
|
|
teodor
National Hazard
   
Posts: 922
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
CaO, 250g - for gas & alcohol drying
Acetylchlorid, 500 ml - for organic synthesis & experiments with dehydration of some metal chlorides
P4O10, 100g - as a drying agent
FeCl3, 250g - anhydrous, for continue experiments with FeCl3/organic layer extractions (I've got some interest in family of [FeCl4]- compounds)
Fe powder < 100um, 500g - just as a source of pure Fe
1-Propanol, 1-Pentanol, 1-Octanol, 1-Decanol (250ml each) - they were missing in my alcohols collection. So, now I have all isomers of 1-4 atomic
alcohols, 2 isomers of penanol, but hexanols-heptanols as well as 9 atoms are still missing.
Salicylic acid, 250g - for salicilate esters and phenol synthesis
|
|
|
chemship1978
Harmless

Posts: 32
Registered: 8-6-2018
Member Is Offline
|
|
Quote: Originally posted by teodor  |
1-Propanol, 1-Pentanol, 1-Octanol, 1-Decanol (250ml each) - they were missing in my alcohols collection. So, now I have all isomers of 1-4 atomic
alcohols, 2 isomers of penanol, but hexanols-heptanols as well as 9 atoms are still missing. |
I've got hexanol, heptanol, sec-octanol, dodecanol, tetradecanol (mirystyl alcohol) and hexadecanol if you were interested in widening your collection

|
|
|
teodor
National Hazard
   
Posts: 922
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
Quote: Originally posted by chemship1978  | Quote: Originally posted by teodor  |
1-Propanol, 1-Pentanol, 1-Octanol, 1-Decanol (250ml each) - they were missing in my alcohols collection. So, now I have all isomers of 1-4 atomic
alcohols, 2 isomers of penanol, but hexanols-heptanols as well as 9 atoms are still missing. |
I've got hexanol, heptanol, sec-octanol, dodecanol, tetradecanol (mirystyl alcohol) and hexadecanol if you were interested in widening your collection

|
Thank you, but my next purchase will probably be some chromium compounds, I need CrO3 for sure - it is waste of reagents to make it from K2Cr2O7 and
H2SO4 as I did before, especially now ... and I am looking for best quantity/price for it (purity is not so important).
Also some "blue" Cr(III), most obvious KCr(SO4)2·12(H2O) but I am doing a lot of experiments with Cr now so probably depending on availability will
get interest in other compounds, the purity is important in this case, as a reference ...
Also I have pain in the ass (and the lungs as well) crystallising Co(NO3)2, so for good price I would probably just buy some ... as well as probably
some other (definitely Cr) metal nitrates, even if I can make them they prefer to crystallise by seed crystals only, so I need few samples for seeding
... the price is important, also I would prefer to buy, let say, 5 different samples 10g quantity each (different metals) than 1 sample 50g.
Well, also I am preparing standard solution for acidimetry/alkalimetry etc, so need some reference compounds with best purity, and I am not sure what
is available on the market. I've made some 0.1N AgNO3 last week using pure Ag which people sell for element collectors , but I also need sodium
oxalate and Mohr's salts (as standards) which I've made by myself but purity ... well, I am not sure how good is it for precision work, I mean I did
crystallisation by myself and now need use it as a reference by weight.
So, if somebody have idea/offers of anything I mentioned, just U2U me.
|
|
|
AvBaeyer
National Hazard
   
Posts: 651
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Ordered some oxalic acid advertised as "100% pure and anhydrous" on Amazon. I wanted to be certain that it was truly anhydrous so I checked the
melting point. It started to melt around 95C and finished at 102C. Even more water than dihydrate given the mp characteristics. The anhydrous form
should melt around 189C. When it sounds to be too good to be true, it usually is. Buyer beware.
AvB
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Many times I have seen chemicals sold as "anhydrous". In most cases it just means that the chemical is a dry powder or free flowing crystalline solid.
I even once saw copper nitrate being sold as anhydrous on ebay, it was just Cu(NO3)2.3H2O, but it was a nice dry solid, while copper nitrate
frequently is moist, due to its hygroscopic nature.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I didn't place any new orders, but my adipic acid finally arrived. Also, my sodium borohydride finally made its way to my lab.
I'm going to be traveling for work for the next two months, but I'm definitely looking forward to getting back into chemistry when I get back.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I purchased 100 grams of arsenic sulfide, As2S3, for the amazingly low price of just $9.99 (appr. EUR 8.50), including shipping. The order arrived
last week and today I tested its purity. I am really impressed about the purity of this product of natural origin. This material easily dissolves in
dilute warm NaOH, and the solution is completely clear, and very pale yellow. No insoluble crap or turbid solution as one normally would expect from
natural minerals.
This opens up relatively safe aqueous arsenic chemistry for a very affordable price. Water-soluble arsenic compounds are quite hard to obtain, so if
you are interested in arsenic chemistry AND you can work cleanly, then I would grab 100 grams of this as long as it lasts.
https://nl.aliexpress.com/item/33017545866.html
If you use this material for experimenting, be careful. This material is TOXIC, especially when it is dissolved!! Avoid reactions with strong
reductors, where H2 can be formed. As a side reaction, small amounts of AsH3 may be formed, besides H2!! So, unless you are absolutely sure about your
safety measures, do not use with zinc or magnesium in acidic solution, or with Al in alkaline or acidic solution, nor with NaBH4!
The seller calls this stuff realgar and also he also uses the word "orpiment". This is not realgar. Natural crystalline realgar is a bright scarlet
red compound, As4S4. This yellow material is orpiment. Natural orpiment may contain realgar as impurity, but fortunately my sample does not contain
that.
[Edited on 16-12-20 by woelen]
|
|
|
itsallgoodjames
Hazard to Others
  
Posts: 276
Registered: 31-8-2020
Location: America Lite
Member Is Offline
|
|
My calcium acetylide has finally arrived from the UK! I'm ecstatic. Hopefully I have time to make copper and silver acetylides this weekend. A few
days ago I also received some potassium permanganate
Nuclear physics is neat. It's a shame it's so regulated...
Now that I think about it, that's probably a good thing. Still annoying though.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I now also ordered 100 grams of realgar, the red arsenic sulfide As4S4. Also from AliExpress for a really low price.
https://nl.aliexpress.com/item/1005001309126232.html
It will take a few weeks before it arrives, but I am not in a hurry. Always good to have a little more of this as well. I now only have 10 grams or so
of this compound (very expensive on eBay).
|
|
|
valeg96
Hazard to Others
  
Posts: 254
Registered: 6-4-2014
Location: Italy
Member Is Offline
Mood: Moodless
|
|
Quote: Originally posted by woelen  | I now also ordered 100 grams of realgar, the red arsenic sulfide As4S4. Also from AliExpress for a really low price.
https://nl.aliexpress.com/item/1005001309126232.html
It will take a few weeks before it arrives, but I am not in a hurry. Always good to have a little more of this as well. I now only have 10 grams or so
of this compound (very expensive on eBay).
|
Can you please keep us updated on that (quality, appearance...)? I've been thinking about buying it ever since it appeared for sale 4-5 years ago, but
never did. I lowkey fear I'll get some half-assed solid filled with sand or soil. I know it's not an ACS chemical but I'd rather not buy a bag of
yellow sand.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
For this one I can say that I was really nicely surprised: https://nl.aliexpress.com/item/33017545866.html
This is really pure, almost lab grade! This is not realgar, but orpiment, As2S3. I really recommend this!
I used it for my newest experiments on my website about arsenic chemistry.
Here follows a picture of the material I have in my hands:

I do not yet know about the realgar I ordered yesterday. I'll tell over here about the quality of that material when I receive it.
[Edited on 23-12-20 by woelen]
|
|
|
valeg96
Hazard to Others
  
Posts: 254
Registered: 6-4-2014
Location: Italy
Member Is Offline
Mood: Moodless
|
|
Yes, that orpiment is exactly what I set my eyes on. There's also some cinnabar that looks really nice on Aliexpress, but is much more expensive (ca.
30€/100g)
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I just did a nice energetics experiment with the orpiment. I crunched some and carefully mixed it with some KClO3. When ignited, this mix burns very
fast. Do NOT inhale the smoke! I only did this experiment at 100 mg scale or so. No weighted amounts, just half a spatula full of orpiment and a
spatula full of KClO3 and carefully mixed with a soft twig. This definitely is not a bag of sand or soil!
[Edited on 23-12-20 by woelen]
|
|
|
valeg96
Hazard to Others
  
Posts: 254
Registered: 6-4-2014
Location: Italy
Member Is Offline
Mood: Moodless
|
|
I don't deal with anything that involves per/chlorates or anything energetic, so there's no way I'd do that. I wish there was a safe and quick way to
turn it into a decent salt or the oxide. Sadly, burning it can't be done at home. I remember seeing a medical report in a toxicology book about a man
who tried to make and sell homemade arsenic pigments in the 1840s and killed off his whole household overnight with the fumes. I assume he did
something like that, as As oxides are fairly volatile and sublime easily.
Edit: your feedback and article convinced me. After like 6 years, I just ordered it myself (the ground up orpiment).
[Edited on 23-12-2020 by valeg96]
|
|
|
Chemcraft
Hazard to Self
 
Posts: 98
Registered: 8-6-2017
Location: Russia
Member Is Offline
Mood: No Mood
|
|
Chemcraft offers arsenic 99% at $40/100 g.
https://chemcraft.su

[Edited on 24-12-2020 by Chemcraft]
|
|
|
| Pages:
1
..
52
53
54
55
56
..
60 |