symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
Collaborative newsletter for ScienceMadness??
By the month like for instance making sodium metal by alcohol catalysis or nitric acid by transition metal salts was finished on 24 Oct link to the
wiki it might make building the wiki easier it would have the current montlth and a point in history on that month or something like that.
I think this will raise awareness and interest in more experiments to further the study in that direction.
[Edited on 18-10-2018 by symboom]
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Seems a bit forced. Some months are very busy while others may have very little to report on.
Edit: An annual report may be more feasible, and it would be a good stepping stone towards the journal.
[Edited on 10-18-2018 by Texium (zts16)]
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
if there are any errors I appologize
Sciencemadness logo centered
Oct 2018 or
2018
Sodium metal has been made using everyday chemicals.
Similar to in a thread in sciencemadness fourm in which potassium is made in an armateur setting an obscure pathway to sodium metal by alcohol
catalysis had been achieved using common chemicals. the chemicals sodium hydroxide tea tree oil and magnesium. Turning something ordinary to
extraordinary is common with armature chemistry.
A turning point and Change in what is possible with so few chemicals.
What else has happened in 2018
Just a thought it's short have more to add this is what I have so far.
[Edited on 18-10-2018 by symboom]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Publishing every month might be a bit ambitious, but a semi-annual newsletter or perhaps an annual State of Mad Science report is an excellent idea.
Hard deadlines will encourage people to get things accomplished and submit their work.
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
I really like the idea of an annual report. It gives a sense of importance and "officialness" (I don't have a word for that). But a group of people
would have to decide what to include: what is relevant and what is not. That might generate some conflict and competition between the members, maybe
in the same way that the race for publication in presitigious journals does.
Or maybe a poll could select what makes it into the annual report? Maybe a poll with many criteria, for example: quality of the post, interest
generated, impact on the amateur community, how successful it was, etc. as perceived by the members.
Maybe a "hot topics" section would be appropriate, where top-voted and recent threads could be rewarded. Like a thread-of-the-month but with some
flexibility in the duration.
Just a few thoughts
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Quote: Originally posted by Heptylene  | I really like the idea of an annual report. It gives a sense of importance and "officialness" (I don't have a word for that).
|
Oh, that's called "flattery."
| Quote: |
But a group of people would have to decide what to include: what is relevant and what is not. That might generate some conflict and competition
between the members, maybe in the same way that the race for publication in presitigious journals does.
|
You'd think that a single editor would be able to handle it.
| Quote: | | Or maybe a poll could select what makes it into the annual report? Maybe a poll with many criteria, for example: quality of the post, interest
generated, impact on the amateur community, how successful it was, etc. as perceived by the members. |
I really think this is the wrong approach. Chemistry isn't a popularity contest; any decison as to what makes it into the final newsletter should be
made on rational grounds by people who are competent chemists.
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
I'd be happy to write up the annual report! If it was to get published in January of each year I should be able to do it no problem, as I have a lot
of free time in winter.
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
Texium (zts16) sounds great I'm glad to see more interest in this.
Jjay great name
The State of Mad Science
What would be in the newsletter??
Experiments?
News about amature chemistry?
There are so many old good threads how about a history section like this month in history. All ideas
[Edited on 19-10-2018 by symboom]
|
|
|
j_sum1
Administrator
       
Posts: 6336
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Significant experiments presented by members
Significant new procedures -- since Nurdrage's progress on the sodium has occupied much of out thoughts and a bit of discussion. (Although I am
not aware of anyone who is actually following his procedure yet.)
Significant postings and procedures in prepub
Changes in availability of chemicals / equipment.
Changes in legislation that affect members.
Some things related to this community:
progress on the spam issue
we had SM member of the year a while back (thanks to aga). Maybe some accolades to individuals.
Highlights from some of the favourite threads: pretty pictures, tour my lab, that kind of thing
Personal developments: for example Tdep's great new (top secret) job doing chemistry for the military. Metacelsus heading off to the UK to
pursue some great opportunities in education. Blagfost26 (not his real name) saying, "to hell with it: if you can't beat the system you may as well
go all-in" and opening a corner store selling sodium perchlorate to card-carrying terrorists.
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
We need to make use of the prepublication forum. http://www.sciencemadness.org/member_publications/index.html hasn't been updated in a long time.
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
Very true thank you for resurecting the thread if any thing is new it should be included.
J_sum_1 has many good ideas along with others thoughts and concerns Voting seems to be the quickest way to Finish a lot of the project.
if there is any disagreement we should have a poll to maintain constructive discourse.
------
Legend
# = denotes needing community input.
@ = denotes needing a vote
&& = Subjected to change as views change
> = Add small excerpt
#Mad Science Reports# 2020
The art and science of amature experimentalism
Quote by Chemetix
Physicists take their superiority from working with first principles and mathematical logic. Chemistry gets its results from the messy chaotic world
of reality
PUBLICATION
@ Vote on favorite publication
--------
PREPUBLICATION
#Most viewed from PREPUBLICATION
Sodium metal - Illustrated Practical Guide
NEW PROCEDURES
Make Sodium Metal with Menthol
It is a great project and achievement for the experienced amateur chemist
A more efficient way to obtain sodium metal heat sodium hydroxide with magnesium in mineral oil above 200 °C, in the presence of a tertiary or
sterically bulk secondary alcohols, like t-butanol and menthol. The yield of this route if done right can be as high as 90-95%, though the process
takes hours to completion.
----
Make Nitric Acid by Thermal Decomposition of Copper Nitrate
Copper sulfate is reacted with calcium nitrate the resulting calcium sulfate is filtered from the solution as copper nitrate remains.
This reaction is a cheap source of nitrogen dioxide, which can be bubbled through water to generate nitric acid.
The gas can also be bubbled into hydrogen peroxide producing a higher yield
--------
--------
HIGHLIGHTS FROM THREADS
--
Chemistry in general
Preparation of elemental phosphorus
(The lesser known reactions)
Cleaned, boiled and dried chicken bones (bone meal can also be used with if used as organic firtalizer) are burned with a bunsen burner on a fireproof
surface and directly heated with the flame until they have turned into white ash.
2g of this bone ash are mixed with 0.5g magnesium powder
or activated carbon powder and 0.5g Diatomaceous earth. The mix is heated and vapors are lead underwater for the vapors to cool and condense to white
phosphorous.
----
low-temperature production of phosphorus,
the most interesting candidates appear to be phosphates of lead, bismuth, and antimony. silver phosphate can be
reduced and yields finely divided metallic silver and phosphoric acid, which is catalytically reduced in the presence of the silver to give free
phosphorus.
lead phosphate is reduced under hydrogen or methane with hydrogen resulting in the highest yields and methane at 50%
The reaction consists of three stages: 1. The Pb3(PO4)2 is heated up to 300C to drive off any existing water.
2. Once the temp hits 300C the hydrogen is turned on and the tempurature slowly raised to 500C. The hydrogen reduces the Pb3(PO4)2 by ripping off the
oxygen molecules and forming Pb3P2, aka lead phosphide.
3. Upon the cessation of evolution of water, the furnace is again slowly raised up to somewhere between 650-800C. According to the patent, small
amounts of PH3 are liberated at around 600C. This makes sense, the Pb3P2 probably starts to break down somewhere around 600C and thus liberates PH3,
which subsequently start to be reduced to H2 and elemental P at around 650C, so basically at the beginning of the reduction temp the phosphine being
liberated is not hot enough to break down.
phosphates can be reduced by hydrogen at temperatures between 300° and 750°C. Lead phosphates are particularly easy to reduce by hydrogen. For
example, pyromorphite, 3Pb3(PO4)2*PbCl2, starts to react at 300°C and is completely reduced at 850°C.
Other possible reducing agents
CaC2 would make a good reducing agent in such a reduction as phosphate reduction. I suspect that sodium polysulfide would work well in a phosphate
reduction. 4Na3PO4 + 2Na2S2 ----> 8Na2O + 4SO2 + P4
Using sodium acetate as a carbon source
fusing Hexasodium metaphosphate with Sodium acetate the mixture liquefies quickly and a strong smell of garlic was released. Sodium acetate acting as
a carbon source and a flux because once the melt was fluid there was a large release of Phosphorus.
Phosphorous from phosphine
phosphine with dimethylchloramine, which reacts to form elemental phosphorus and dimethylammonium chloride (as
reported here: http://pubs.acs.org/doi/abs/10.1021/ic50067a009).
___________
Organic Chemistry
Reagents and Apparatus Acquisition
Repurposed gear
tour my lab
#
Biochemistry
Technochemistry
Nitric acid from ammonia Oswalt style (without platnium)
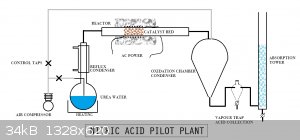
Reactant used: Urea and Distilled water
Catalysis NiO or CoO in support bed of Sand or red house brick
Devices: Absorption tower (converted 2L sep. funnel water with broken glass to increase surface area. Quartz Catalysis tube 8mm ID. A condenser. Air
compressor.
nichrome wire heating element with insulation.
This process uses a NO2 generator using urea as a source of ammonia and oxidising with air using nickel oxide or cobalt oxide on sand or red house
brick as a catalyst A condenser is used to dry the ammonia/air stream. The ammonia air mixture is fed into the reaction zone, the nice red glow is
transmitted up the quartz leads into a Quartz tube as reaction chamber. The reactor tube runs into the reaction chamber which admits air via the
custom condenser fitting. The condensate runs into a reservoir where excess gasses run to an absorption tower
exhaust gasses pass into a vertical anti suck back column before running along a small tube to the base of the tower.
pretty pictures
#
#
---------
FORUM MATTERS [may need revision]
Ever wonder how many members are in sciencemadness
We have 288131 mad scientist members.
Spam problem eliminated
With the change of registration to email only (date start needed) sciencemadness is finally is not having problems with constant spam alot of hard
work was going into avoiding this last resort but the board has benefited from the change.
With even a span killer designated to take on the problem before and a subform named Test Forum with the Forum Moderator as streety
It was a Test area for testing spam prevention mechanisms.
The message everyone sees is the reasoning.
The management apologizes for the inconvenience, but it is necessary to keep abusive users and bots out.
#
PERSONAL DEVELOPMENTS
Tdep's great new (top secret) job doing chemistry for the military.
Metacelsus heading off to the UK to pursue some great opportunities in education.
Cou's business venture of fragrance compounds
#
LEGAL AND CHEMICAL AVAILABILITY ISSUES
Changes in availability of chemicals / equipment.
#
Changes in legislation that affect members.
#
------
Recognition of collaberation (subjected to change as more help add)
J_sum1
Heptylene
Cou
clearly_not_atara
Texium (zts16)
_______________________________
Sciencemadness logo centered
#Mad Science Reports# 2020
The art and science of amature experimentalism
PUBLICATION
PREPUBLICATION
PROCEDURES AND EXPERIMENTS
HIGHLIGHTS FROM POPULAR THREADS
PERSONAL DEVELOPMENTS
LEGAL AND CHEMICAL AVAILABILITY ISSUES
FOURM MATTERS
SECRET SANTA
(It looks like it comes to 10 pages)
______
Hope to have this published by January 1 and pass off to an admin for finalization to be added to member publications or in this case members joint
publication
Todo list
Add excerpt of toluene --> benzaldehyde
Update HIGHLIGHTS FROM POPULAR THREADS
@voted items of members
Title of newsletter
Publication
prepublication
Other
Request members add to
PERSONAL DEVELOPMENTS
LEGAL AND CHEMICAL AVAILABILITY ISSUES
Figure out the best way to make this.
This is a rough draft
[Edited on 22-10-2020 by symboom]
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
"Journal of Amateur Chemistry"?
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
We had had another similar thread proposing a free journal for home chemistry, with a poll for the name -- I liked Acta Chemica Libera -- but
this effort is much more specific, with no submissions, just a report on what happens on the forum.
So I think the better name is just Mad Science Reports or something else containing "ScienceMadness" or "Mad Science". It also sounds more
tractable because honestly neither I nor anyone else had the time to run a journal apparently (and I have less time these days).
Also I would suggest putting the "journal" out every two months rather than every month, truly interesting results don't come around that often and it
would save half of the work.
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
Quote: Originally posted by Texium (zts16)  | Seems a bit forced. Some months are very busy while others may have very little to report on.
Edit: An annual report may be more feasible, and it would be a good stepping stone towards the journal.
[Edited on 10-18-2018 by Texium (zts16)] |
Change to annual instead of monthly
Totally agree.
__________
I think I'll add the biggest strides that I can think of
Adioazide azide c2n14 covers many compounds and other projects
C2N14
excerpt of synthysis by Engager And Tdep
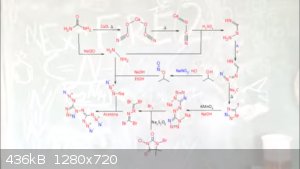
calcium cyanamide
involves heating a mixture of calcium oxide and urea at 120-350 °C which gives calcium cyanate, followed by calcination at 7-900 °C to give calcium
cyanamide
Aminoguanidine prepared from calcium cyanamide and hydrazine sulfate. Reaction produces impure aminoguanidine, which is then converted to
aminoguanidine bicarbonate, for easier purification
5-AminoTetrazole through the action of nitrous acid and aminoguanidine
Disodium 5,5'-AzoTetrazole is produced by the reaction of KMnO4 and 5-AminoTetrazole
Isocyanogen Tetrabromide
Bromine rips apart the tetrazole groups to form an obscure molecular motif, needed to make the famed C2N14 energetic compound
C2N14 (soulble in acetone)
The distilled water is added to the isocyanogen tetrabromide this is so the excess bromine is pulled into the water
Sodium thiosulfate is added to nutrilize the bromine
The thiosulfate solution above the solid is remove then washed with distilled water then acetone is added which the isocyanogen tetrabromide
redesolved in the acetone
This is placed in an ice bath to 0C
sodium azide desolved in water it is added to the cyanogen tetrabromide acetone solution.
The solution is then allowed to return to room temperature
Water is added to precipitate the product. This is then put back into the ice bath to precipitate the crystals.
-----
Hydrazine synthysis
solution of 10% sodium hypochlorite is added to solid sodium hydroxide and the mixture is kept cold during the addition. Meanwhile, a solution of
urea with a few hundred mg of gelatin is prepared. The two solutions are mixed and allowed to fully react, a voluminous foam will appear. After they
have reacted, MEK is added to the solution and the azine is processed as previoulsy described.
----------
Sodium azide
Isopropyl nitrite reacts with hydrazine hydrate and sodium hydroxide to form sodium azide
Isopropanol nitrite
Concentrated hydrochloric acid is slowly dripped onto sodium nitrite suspended in isopropanol, generating nitrous acid which reacts with the alcohol.
--------
synthesis and highlights those
Phosphorous
Sodium
Tetrazide C2N14
________________
Organic Chemistry
Many amateurs have attempted to make acetic anhydride at home due to its tremendous usefulness in organic chemistry. Only a few have succeeded, though
a write-up of the preparation of acetic anhydride from sulfur, bromine, and anhydrous sodium acetate was reported by Magpie.
Another more accessible method involves the reaction of acetyl chloride and anhydrous sodium acetate.[1]
A preparation that is as of yet not well-researched involves the dry vacuum distillation of anhydrous zinc acetate, which gives off acetic anhydride
and converts to basic zinc acetate. If achievable, this would mean a route to acetic anhydride that only requires glacial acetic acid and cheap zinc
metal.
_______
Preparation of Acetic Anhydride
by Magpie
8CH3OONa + S +3Br2 = 4(CH3O)2O + 6NaBr + Na2SO4
anhydrous sodium acetate and set aside in a covered beaker. Weigh out 3.5g (0.11 moles) of sulfur in a beaker and set aside. Have available 20 mL
(0.39 moles) of dried Br2.
Mix the bromine and sulfur together
anhydrous sodium acetate
bromine is the limiting reagent. The sulfur, except that released as SO2, is recycled in situ.
Vacuum Distillation
Set up for vacuum distillation of the Ac2O using the 3-neck 500mL RBF. Place an insulating blanket over the RBF. With a vacuum of 23”Hg (absolute
pressure = 178 mmHg) the Ac2O comes over at about 72°C. Yield of the crude Ac2O will be ~30 mL.
E. Simple Distillation
Set up for simple distillation of the crude Ac2O at atmospheric pressure. Use a 50 mL RBF as pot. Collect 2 cuts. The first cut (~15 mL) will come
over at about 128-135°C. The 2nd cut (~10 mL) will come over at 135-143°C. Literature value for the bp of Ac2O is 140.0 °C. Yield, based on the
10mL of cut 2 and the amount of Br2 charged, is ~27%.
Biochemistry
Radiochemistry
Ion chamber see ions with dry ice and isopropanol
Reagents and Apparatus Acquisition
Repurposed gear
tour my lab
#
_____
References used
In prepublication as Acetic anhydride by magpie
http://www.sciencemadness.org/talk/viewthread.php?tid=15021
Capsasin extraction
http://www.sciencemadness.org/talk/viewthread.php?tid=5329
Extract DNA from strawberries
https://m.youtube.com/watch?v=araeHtN_3Lk
[Edited on 23-10-2020 by symboom]
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
Sulfur trioxide
The preparation of this compound is extremely dangerous and should only be attempted by chemists with experience working with hazardous volatile
reagents.
SO3 can be made in low yield through the pyrolysis of sodium persulfate, first forming ozone and then SOSO3. A catalytic amount of 100% sulfuric acid
is required.
Nearly the same reaction takes place when sodium bisulfate is very strongly heated, first evolving water, forming sodium pyrosulfate, which then
decomposes above 460 °C to sodium sulfate, releasing SO3.
There are reports that heating sodium pyrosulfate with concentrated sulfuric acid to 150°C results in SO3 and bisulfate.
Heating iron(II) sulfate at 700 °C with carbon yields iron(III) oxide, sulfur dioxide and sulfur trioxide. The same reaction also works with
iron(III) sulfate, at a much lower temperature, 480 °C, and produces mostly sulfur trioxide.
Pyrolysis of copper(II) sulfate above 560 °C yields sulfur trioxide and copper oxide. If the temperature gets lower, copper sulfate will reform.
Aluminium sulfate also works, though the decomposition temperature is slightly higher.
Adding phosphorus pentoxide to extremely concentrated sulfuric acid will release sulfur trioxide, which can be extracted via distillation.
Metaphosphoric acid can also be used instead of the pentoxide.
Roasting calcium sulfate with silicon dioxide (very fine sand can be used) at 1000 °C for 1 hour yields calcium silicate and sulfur trioxide. Adding
small amounts of chromium(III) oxide or tungsten(IV) oxide improves the process.[3]
In industry sulfur trioxide is produced via the contact process. Purified sulfur dioxide is mixed with dry air and injected through a bed of V2O5
catalyst heated to temperatures between 400-600 °C (the optimum temperature is 450 °C), at 1-2 atm. The resulting hot sulfur trioxide is
recirculated and passed through more layers of catalyst to increase the yield. The gaseous SO3 is cooled by passing it through a heat exchanger and
can be collected if required. The condensed trioxide is dissolved in concentrated H2SO4 in the absorption tower to form oleum.
Sulfur trioxide can also be obtained by oxidizing sulfur dioxide in the presence of several metal oxides, such as copper(II) oxide at high
temperatures[4] or chromium(III) oxide (at temperatures between 180-400 °C).[5]
Sulfuric acid
How to make sulfuric acid by electrolysis of copper sulfate using an inert anode. Copper sulfate is very easy to obtain in large quantities at
gardening and hardware stores and provides a convenient route to sulfuric acid if the appropriate anode can be obtained.
Warning: This should be done in a well-ventilated area as hydrogen gas build up is explosive. Copper sulfate is toxic and sulfuric acid is corrosive,
wear gloves when handling them.
The procedure is extremely simple, just get a copper sulfate solution, insert two electrodes and run a current through them. The anode, the positive
electrode, must be an inert material that can withstand extremely oxidizing conditions. Very few materials meet this condition, platinum, lead
dioxide, and carbon among them. Other metals, even stainless steel, are quickly destroyed under these conditions and cannot be used. The cathode, the
negative electrode, is exposed to reducing conditions so the metal requirements are must less stringent. Copper is the best choice here since it has
high electrical conductivity.
When applying power, the current should be adjusted so that corrosion at the positive terminal and bubbling at the negative terminal are both
minimized. The bubbling at the negative terminal is hydrogen production and that's wasted energy that should have gone into reducing the copper
sulfate.
After the solution has gone clear, filter off the particles and the clear filtrate is dilute sulfuric acid that can be boiled down to obtain
concentrated sulfuric acid. It will have trace amounts of metals but for most purposes this is not an issue.
Keep in mind that it will take a long time for the solution to go clear. if after filtering it's still blue, you'll need to keep electrolyzing it.
You'll need over 60 amp hours of current per mole of copper sulfate, and usually more since the process is not very efficient
_____
Make Sulfuric acid (metabisulfite/oxidizer method)
sulfuric acid from sodium metabisulfite, hydrochloric acid and an oxidant such as hydrogen peroxide or nitric acid.
Warning: The procedures in this video produce large quantities of toxic gases and deal with highly corrosive acids. All work must be performed in a
fume hood with proper safety equipment. And all apparatus must be glass to withstand the acids.
Sodium metabisulfite upon reaction with acid will generate sulfur dioxide. This provides a convenient source of sulfur dioxide that is easier to
handle than burning sulfur, but it is acceptable if you want to go that route. You'll just need to build a sophisticated gas capture and scrubbing
system so the sulfur vapors and soot don't clog your tubes, poison your air and possibly burn down your workspace.
Sulfur dioxide is converted into sulfuric acid by reacting it with an oxidizer in water. In this case either hydrogen peroxide or nitric acid.
Industrially, sulfur dioxide is reacted with oxygen over a catalyst to make sulfur trioxide. This is cheaper but extremely difficult to do safely for
the home chemist so the metabisulfite/oxidizer method is used instead.
You may use potassium metabisulfite instead of sodium metabisulfite. Both are used by home brewers to sterilize winemaking and beermaking mixtures.
It's also used for homemade dyeing processes. Therefore it is very easy to obtain the metabisulfites without the need for expensive shipping fees or
licenses.
The oxidizers must be present for this reaction to work. You cannot simply use water or you'll just make sulfurous acid which decomposes on heating
and is useless for the reactions that sulfuric acid is intended for.
In future videos, we may show other methods of sulfuric acid production including sulfur trioxide based methods so please subscribe!
Make Sulfuric Acid by the Copper Chloride Process
Take a solution of copper (II) chloride and bubble sulfur dioxide into it until most of the copper (II) chloride is converted into copper (I)
chloride. This reaction also converts the sulfur dioxide into sulfuric acid and produces hydrochloric acid. Now the copper chloride can be regenerated
by bubbling air into the mixture until the copper chloride dissolves again. This cycle can be repeated. When you want to isolate the sulfuric acid you
forgo air infusion for that step and filter off the preciptated copper chloride. Then you distill off the hydrochloric acid and water from the
filtrate. The sulfuric acid left behind will precpitate out most of the remainig copper salts and when filtered will give you relatively pure sulfuric
acid with some water and minor copper contamination. The copper chloride can be recombined with all the hydrochloric acid from before and the cycle
repeated.
________
Sulfuric acid water and sulfur (electrobromine process)
make sulfuric acid from sulfur and water using electrolytically generated bromine as the catalyst.
Warning: Bromine and sulfur bromides are volatile and toxic. This might be done outside or in a fume hood.
We start with 16g of sulfur in a 250 ml beaker. We install 5 lantern battery carbon electrodes for the anode and a copper wire for the cathode. To
this setup we add 200mL of 5M hydrobromic acid. The copper cathode is readjusted to be as physically close to the top of the electrolyte as possible
while still being immersed in it. A current is then applied of less than 2 amps. The electrolysis is performed for 40 hours with occasional stirring
and addition of water to make up for evaporative loses.
Whats happening is the electrolysis converts hydrobromic acid to bromine and hydrogen. The hydrogen bubbles away and the bromine reacts with sulfur to
produce disulfur dibromide. This in turn reacts with more bromine and water to produce sulfuric acid and hydrobromic acid.
At the end of the run, the solution is filtered and then refluxed for 30 minutes to drive the conversion to completion. The solution is then distilled
to first recover water, then hydrobromic acid and finally sulfuric acid.
References
Make Sulfuric Acid (Copper Sulfate Electrochemical Method)
https://m.youtube.com/watch?v=5dUSF9Gl0xE
Sulfuric acid water and sulfur (electrobromine process)
https://m.youtube.com/watch?v=6ms6xbPhdVs
Make Sulfuric Acid by the Copper Chloride Process
https://m.youtube.com/watch?v=l2AkVYxDSKc&t=13s
Make Sulfuric acid (metabisulfite/oxidizer method)
https://m.youtube.com/watch?v=okvvD3-DF9U
-------
Sulfur trioxide
http://www.sciencemadness.org/smwiki/index.php/Sulfur_trioxi...
Sulfur Trioxide and Oleum, by GARAGE CHEMIST [Publication]
http://www.sciencemadness.org/member_publications/SO3_and_ol...
--------
Ostwald style nitric production [Technochemistry]
http://www.sciencemadness.org/talk/viewthread.php?tid=71282
---------
Preparation of elemental phosphorus [Prepublication/Chemistry in general]
http://www.sciencemadness.org/talk/viewthread.php?tid=65
Preparation of Acetic Anhydride
[Prepublication/Organic chemistry]
http://www.sciencemadness.org/talk/viewthread.php?tid=15021
--------
C2N14 (azidoazide azide)
https://m.youtube.com/watch?v=uNhVK-2mh6w
------
>>>>>Possible adding
Maybe add a Building a Home Lab Article
http://www.sciencemadness.org/smwiki/index.php/Laboratory
10 Chemical reagents
Sulfuric acid
Hydrochloric acid
Nitric acid
Sodium Hydroxide
Sodium Bicarbonate
Reducing agent (Zinc, Aluminum)
Oxidizer (Sodium Hypochlorite, Hydrogen Peroxide)
Desiccant (Calcium Chloride)
Glassware
Safety
A building a fumehood
Clean up
Halogen clean up by thiosulfate
Acid spill by sodium bicarbonate
Poison antidotes
_______________
Legal and regulations
Chemistry from both sides of the table
http://www.sciencemadness.org/talk/viewthread.php?tid=156366
http://www.sciencemadness.org/smwiki/index.php/Regulatory_qu...
Texas lab glassware ban
_______
Progress so far
This is the information I want to add
At the moment I'm compiling it onto
My laptop with abiword my free trial of word ended in my laptop. It saved with a .doc Microsoft word compatible format.
I plan to do a poll after i complete the final draft
Which will say
Is the mad science report newsletter ready to be added to prepublication
Final draft posted on the poll
>Yes
>No I would add
>No I would remove
For it to be ready
[Edited on 23-10-2020 by symboom]
[Edited on 23-10-2020 by symboom]
|
|
|
BrainAmoeba
Harmless

Posts: 15
Registered: 12-7-2020
Location: It depends...
Member Is Offline
Mood: Poisoned
|
|
Hello everybody,
Creating a journal/magazine may be a very challenging task.
It will require a lot of work but I feel it is worth it.
If I can be helpful in anyway (e.g. graphical design, reaswrch, etc.), I will help.
Thank you for your effort.
P.S. It can be named: Journal of Mad Science, or something. I am not the best in creative titles.
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
@BrainAmoeba
Hey graphical design sounds great and would really make this work
For research this is a completion of sciencemadness and many discussions are based on articles on other web sites. If there is something you feel like
would be a great addition go for it and post pieces of it here.
Statement from other thread
http://www.sciencemadness.org/talk/viewthread.php?tid=156376
clearly_not_atara
We want typesetting!
I'll try to mock something up this weekend if nobody gets to it
before me.
__________
A note of encouragement of collaberation
The only thing I worry about is this project dying in the process as one person waits for the other person and doesn't want to put out the samething
most of the time you will be the only one and we could always vote if needed. Whatever gets this to it's goal before January. Unfortunately that
happens a lot with collaboration projects. It happened with the collaboration book of chemistry, and many others it is hard to do. Don't be
discouraged if any one wants to try it have a hand at it.
[Edited on 28-10-2020 by symboom]
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Glassware is not illegal in texas anymore. That law was repealed in 2019
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
Thanks for that update that will be added to Legal and regulations section
Also read a post by
Quote: Originally posted by gerrockium  |
hello guys, first post here, i've been browsing the forum several years ago, the idea of the periodic publication journal brough my attention
and im planning on being more active user.
|
This is some great news and what I intended it to help with engagement so amature chemistry can grow more with the newsletter then the journal can be
build on this momentem
I don't want to stifle innovation.
[Edited on 29-10-2020 by symboom]
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
tetrazole
Picture excerpt from
http://www.sciencemadness.org/scipics/?C=S;O=A
Missing sodium thiosulfate nutralization of bromine
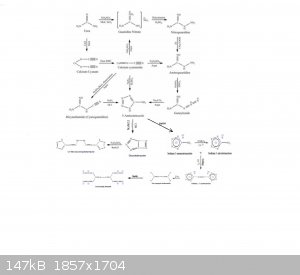
[Edited on 31-10-2020 by symboom]
[Edited on 31-10-2020 by symboom]
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
here is what I have so far
Attachment: mad science report.doc (84kB)
This file has been downloaded 454 times
|
|
|