Whathappensif
Hazard to Self
 
Posts: 53
Registered: 9-7-2020
Member Is Offline
|
|
Simple exploding wire apparatus for nanopowders
Has anyone set up a high power electrical discharge apparatus to make nanopowders?
According to this article, you need a current density of 104 - 106 A/mm2.
https://en.wikipedia.org/wiki/Exploding_wire_method
So doing some sums:
Resistivity Aluminum = 2.65e-8 Ω·m
Wire diameter = 0.1mm
Wire area = 7.9e-9 m2
Wire length = 10mm
Wire resistance = 0.034 Ω
Obviously all aluminum comes with a thin oxide coating, so the resistance will be higher than what I calculate
12V lead acid battery internal resistance = 0.01 Ω
Therefore discharge current = 274A
Current density = 34930 A/mm2 (exceeds the 10e4 value for the production of nano powders)
However, it seems this exceeds the maximum 10s discharge current of a lead acid battery, which is 25A.
https://electronics.stackexchange.com/questions/130580/what-...
So the question is whether a lead acid battery can provide 274A of current for <1s, and whether it can do so for what is a micro-batch process, for
an extended duration, without killing the battery.
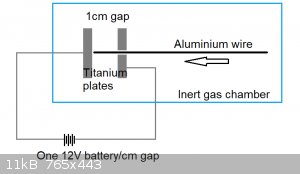
My idea is to extend a thin 0.1mm diameter wire through a holed plate (or even a titanium tube), offset from another titanium plate by a distance of
1cm. When the wire touches the other titanium plate, it completes an electrical circuit and in theory 274A of current gets dumped into it.
This apparatus would require:
1. Inert gas chamber + inert gas supply
2. Titanium plate x2, or titanium plate + >0.1mm inner diameter titanium tube
3. 12V lead acid battery
4. Advancement mechanism for the aluminum wire, which may initially be by hand
All are pretty easy to build/acquire, except for the inert gas supply.
|
|
|
wg48temp9
National Hazard
   
Posts: 786
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Your main problem is you need sufficient voltage to produce an arc. When the wire melts and forms a line of molten metal sphere an arc is required to
continue the current flow. I doubt 12v would be sufficient to produce the arc. As per your wiki link its usual done with a charged capacitor and from
my knowledge the voltage is usually in thousands of volts.
It may be possible to use an inductance in the circuit to strike and maintain the arc but I suspect it would be large (tens of kilo) if it has
sufficient storage capacity and low resistance.
Car batteries are specified to have cranking currents of =>800A but at reduced voltage but you may need a lot of them in series.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
Sulaiman
International Hazard
    
Posts: 3720
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
For a similar project I used the flash units from disposable cameras.
You can use multiple units in series and / or have multiple capacitors in parallel.
If you have access to old disposable cameras with flash units.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Whathappensif
Hazard to Self
 
Posts: 53
Registered: 9-7-2020
Member Is Offline
|
|
OK, good point about needing to maintain the arc. Do you think keeping an electrical current flowing through the melted wire is essential to
generating nanopowders? Otherwise the surface tension of the molten droplets will cause the metal to condense into micron or mm spheres? What is the
resistance of the molten metal channel?
I found this article about making nanopowders by electrical explosion of wires. The key bit appears to be this:
| Quote: |
The tungsten and aluminium wires with diameter d = 0.2...0.3 mm and length l = 40...200 mm were used in the experiments. Electrical
explosion of wires was carried out under conditions of “fast” explosion with an arc stage or with infinite current pause when almost
all accumulated in capacitor energy was consumed by wire before explosion. Energy parameters of EEW were regulated with change of charging
voltage and geometric characteristics of exploding wires. The specific electrical energy input in the wire (е) was changed from
0.4 to 1.8es (es is the sublimation energy of the wire material); the energy of the arc stage (еa) – (0.7...1.7)еs. O.
Nazarenko Parameters of electric circuit: capacitance С = 2.25 μF; charging voltage U = 15...30 kV; inductivity L = 0.58 μH.
|
Attachment: nanopowders_by_electrical_explosion_of_wires_2007.pdf (215kB)
This file has been downloaded 497 times
|
|
|
wg48temp9
National Hazard
   
Posts: 786
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by Sulaiman  | For a similar project I used the flash units from disposable cameras.
You can use multiple units in series and / or have multiple capacitors in parallel.
If you have access to old disposable cameras with flash units. |
You can use the mains power from a cooker outlet if you bypass the breakers or have an old fuse type distribution board. Be careful not to blow the
line fuses. You can take pics of the exploding wire using the bulb setting on a camera. The explosion propels spinning blobs of molten metal some then
spilt in two. You can also use the filament/electrode from a florescent tube. It produces a very bright flash.
You can explode the wire in water and it produce's a cavity which then collapses. That can break a small glass containers. That produces some
powdered copper and small blobs of copper. It may be a simple (no inert gas required) method to start experimenting with. Perhaps paraffin oil could
be used for aluminium.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
wg48temp9
National Hazard
   
Posts: 786
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
| Quote: | Quote: Originally posted by Whathappensif  |
The tungsten and aluminium wires with diameter d = 0.2...0.3 mm and length l = 40...200 mm were used in the experiments. Electrical
explosion of wires was carried out under conditions of “fast” explosion with an arc stage or with infinite current pause when almost
all accumulated in capacitor energy was consumed by wire before explosion. Energy parameters of EEW were regulated with change of charging
voltage and geometric characteristics of exploding wires. The specific electrical energy input in the wire (е) was changed from
0.4 to 1.8es (es is the sublimation energy of the wire material); the energy of the arc stage (еa) – (0.7...1.7)еs. O.
Nazarenko Parameters of electric circuit: capacitance С = 2.25 μF; charging voltage U = 15...30 kV; inductivity L = 0.58 μH.
|
At 20kV thats about 450J. To get that amount of energy out of a car battery requires about 37.5A flowing for one second but its required in say 10ms
so it would have to be 3,750A. It could be 1ms. 12 V cannot push that much current thru a thin bit of wire or an arc or perhaps not the stray
inductance in 1ms.
PS Sorry I screwed up the quote formatting.
[Edited on 7/11/2020 by wg48temp9] |
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
Junk_Enginerd
Hazard to Others
  
Posts: 251
Registered: 26-5-2019
Location: Sweden
Member Is Offline
|
|
Quote: Originally posted by wg48temp9  | Your main problem is you need sufficient voltage to produce an arc. When the wire melts and forms a line of molten metal sphere an arc is required to
continue the current flow. I doubt 12v would be sufficient to produce the arc. As per your wiki link its usual done with a charged capacitor and from
my knowledge the voltage is usually in thousands of volts.
It may be possible to use an inductance in the circuit to strike and maintain the arc but I suspect it would be large (tens of kilo) if it has
sufficient storage capacity and low resistance.
Car batteries are specified to have cranking currents of =>800A but at reduced voltage but you may need a lot of them in series.
|
A fat inductor would easily maintain an arc at 12 v, though perhaps not 10 mm, but a quarter or third of that may work. For a 10 mm current sustained
arc, I would expect to need at least 40 V. Combine that with ~10 mH and you should be OK. But then you may run into issues with the current rising too
slow instead...
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
You may have already read it, but if not you may find the following article by Steve Hansen interesting:
http://www.belljar.net/Exploding_Wires.pdf
Among other things, it describes the requirements for doing this, along with a circuit.
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
vanBassum
Hazard to Self
 
Posts: 66
Registered: 16-4-2019
Member Is Offline
|
|
Hello,
So I might be able to help you with this. I don't have an inert atmosphere but I can try things out in the open air. If I understand your
requirements:
- High enough voltage to bridge the gaps that are created when the metal 'melts'.
- High enough current to vaporize the metal wire. (34930 A/mm2)
- Metal wire
- Material: Al
- Diameter: 0.1mm
- Area: 7.9e-9 m2
- Length: 10mm
- Resistance: 0.034 Ω
The combination of current and voltage is something that won't be easy to do with batteries.
So I will suggest to use capacitors.
You need to overcome the resistance of the complete circuit.
Capacitor internal resistance + resistance of wires used to connect everything + resistance of the 'exploding' wire.
All this has also some inductance, this will resist the change in current.
Therefore, when the capacitor is connected the current needs some time to rise.
Meanwhile, the capacitors are discharging while the current is rising.
Now there will be a point where the wire melts and the circuit is broken, you want to reach a high enough current before that point.
This can all be calculated using some differential equations.
Search for "RLC circuit discharge" or something like that.
I do have 3 big capacitors:
- 4700µ @3x => 14.100µF
- 350V @3x => 350V
- 35mOhm @3x => 11.67 mOhm
If you are interested I could do a couple of discharges trough some small wire at different voltages.
|
|
|
RogueRose
International Hazard
    
Posts: 1595
Registered: 16-6-2014
Member Is Offline
|
|
I think a car battery will be fine to produce the amperage in most cases. I'm a little confused as to the length of time you need for the discharge,
you say something about "exceeds the maximum 10s discharge current of a lead acid battery, which is 25A." I'm not sure what you are saying, maybe the
max discharge for 10 seconds is 25A? That is certainly not true.
You can easily do full current of 250-400+ amps (depending on battery) for 10 seconds in most batteries.
You have other options as well such as the lithium ion emergency jump start packs or even the traditional lead acid ones (these have a 17 or 18AH
battery usually and they can put out some serious amperage for their size).
You can also run a couple of the emergency battery packs in series to get the higher voltage to sustain an arc.
|
|
|
Vomaturge
Hazard to Others
  
Posts: 286
Registered: 21-1-2018
Member Is Offline
Mood: thermodynamic
|
|
I'm a bit sceptical. Wikipedia doesn't say the parameters for making nanoparticles, just the levels to create an "exploding wire" in general. 10^4 to
10^6 A/mm^2 only sounds like 2 orders of magnitude difference, but for any given material the power density will vary by 4 orders of magnitude. The
duration of the process is between 10^-8 and 10^-5 seconds.
I'll try to find the real parameters for making nanoparticles in a little while, 10 minutes, maybe a week.
For now, Ill just say that a 100um 1cm aluminum wire weighs maybe 0.2 milligrams, based on cross sectional area density and length. Aluminum has a
specific heat capacity of about .9j/gm-K and a heat of melting of about 400 j/gm. Assuming a temperature rise of 500K to melt it, that means about .18 j will have to be delivered to even melt the wire completely. With
the power output of your theoretical setup, the wire will be partially solid until 5.5*10^-5 seconds, missing the very slowest time criterion. And
that's before we add in the rise in resistance that happens when a metal gets hot.
I'm ignoring the 25kwh/kg requirement since that's probably as variable as the time and current, but if that rule applies, that slows everything down
by another 2 orders of magnitude.
I now have a YouTube channel. So far just electronics and basic High Voltage experimentation, but I'll hopefully have some chemistry videos soon. |
|
|
densest
Hazard to Others
  
Posts: 359
Registered: 1-10-2005
Location: in the lehr
Member Is Offline
Mood: slowly warming to strain point
|
|
If anyone is still working on this, here's what I've seen about exploding wires: You may already know this
Note: Some types of exploding wares are considered war-level munitions. An exploding wire can act as a detonator.
Fast rise time is key i.e. a few microseconds.
While the theoretical energy necessary to explode the wire is small, in order to supply it quickly enough the equipment has to be capable of supplying
much more unless it is extremely carefully designed.
Alternatively a pulse forming network which compresses a long low-power pulse into a short high-power pulse can work. That really gets into the
munition category.
HIgh voltage ( > 200V up as high as 5000V or more) is necessary.
Relatively small inductances will slow the rise down.
Large electrolytic capacitors are made by coiling long pieces of aluminum foil = large inductance
Series resistance of caps can be large. Even a few ohms can be too much.
Multiple smaller caps in parallel work better.
Small diameter & long connecting wires have significant inductance.
Switching the power on without destroying the switch is a real pain.
Either a physical switch like a relay (fused contacts soon)
or a truly huge SCR that can stand the very high pulsed current (most that are big enough have slow turn-on i.e. milliseconds)
Some people use a xenon flash tube as a switch.
And the shock wave can permanently damage your ears
Good luck!
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Piffle. Want to explode wires? Nothing to it.
Couple of car batteries. Heavy gauge Copper (or Aluminum) wire. Bell housing, and vacuum pump.
Common throw switch.
Bolt your Heavy Gauge wire across massive terminals inside a vacuum chamber. Pull a fairly hard vacuum, and flip the switch. Zapp!
Several inches of heavy gauge wire will be vaporized instantly, attended by a brilliant flash of light.
Under ordinary pressure conditions, our soupy atmosphere acts as a serious heat sink. Dispersing and slowing heating. Eliminate that heat sink, by
creating a vacuum, and metals become easy to "explode".
I've observed both the capacitor and vacuum methods. The capacitor method is dangerous and difficult to achieve. Car Batteries are safe, cheap, and
you can borrow a few extras if need be.
|
|
|
Texium
|
Thread Moved
29-11-2023 at 13:38 |