| Pages:
1
..
34
35
36
37
38 |
beta4
Hazard to Self
 
Posts: 56
Registered: 3-2-2019
Member Is Offline
|
|
Yesterday I dropped the stirbar in the sink while washing a flask.
|
|
|
itsallgoodjames
Hazard to Others
  
Posts: 276
Registered: 31-8-2020
Location: America Lite
Member Is Offline
|
|
A few weeks ago I was putting a rubber bulb on a glass pipette. this is about the millionth time I've done it without any issues, but this time the
pipette decided to shatter. That same day, I accidentally broke my last thermometer.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
I do that all the time... I must have lost two dozen stirbars alone in the various toilets in my appartments so far.
When the stirbars start to become scarce, I usually screw the syphon off from the sink and find a few sticking to the metal 
But the toilet? Usually I notice later that my amount of stirbars has decreased and then I remember how I flushed some lye down the drain... and my
stirbar(s).
Well, I hope they get happy in the canalisation, and as in my language we call them actually "stir fishes"(Rührfische), maybe they are really happier
in an aquatic environment?
When I think of this, I like to imagine them as a happy swarm of "stir fishes" of various small to medium sizes, and they are swimming happily
together through the sewerage, and that makes me smile like an idiot 
I know they don't miss me, but often I think "damn I need a stirbar 20 right now, but the last one has escaped down to the others!", and then I miss
them somewhat 
|
|
|
outer_limits
Hazard to Others
  
Posts: 139
Registered: 3-3-2020
Member Is Offline
Mood: hybridized
|
|
My largest one went to shower drain with 300ml of strong NaOH solution. I'm almost sure that I could retrieve it but I would need to break the
ceramics on the floor to get it.
Rest my friend, you served me very well! I hope you will get a chance to stir something toxic or caustic as you loved to!

|
|
|
MidLifeChemist
Hazard to Others
  
Posts: 192
Registered: 4-7-2019
Location: West Coast USA
Member Is Offline
Mood: precipitatory
|
|
My plan is to have only 1 stirbar. Then maybe I'll treat it like gold.
|
|
|
Texium
Administrator
       
Posts: 4618
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Trust me, you’ll want more.
You’ll need different sizes if you work with various sizes of glassware, and you’ll want to have one for nasty stuff that stains, along with some
that are reserved for stirring cleaner solutions.
|
|
|
itsallgoodjames
Hazard to Others
  
Posts: 276
Registered: 31-8-2020
Location: America Lite
Member Is Offline
|
|
Quote: Originally posted by Texium (zts16)  | | Trust me, you’ll want more.
You’ll need different sizes if you work with various sizes of glassware, and you’ll want to have one for nasty stuff that stains, along with some
that are reserved for stirring cleaner solutions. |
I second this. You'll definitely want more than one
|
|
|
TriiodideFrog
Hazard to Others
  
Posts: 108
Registered: 27-9-2020
Member Is Offline
|
|
You can replace stir bars with paperclips, but I would highly not recommend that as they can get noisy and oxide that form on them can contaminate
your solution.
|
|
|
TriiodideFrog
Hazard to Others
  
Posts: 108
Registered: 27-9-2020
Member Is Offline
|
|
Yesterday, I was holding a beaker which contained acid inside when it just broke. The acid spilled everywhere on my workbench, but luckily it was
quite mild. I think the beaker cracking had to do with me putting the beaker in the microwave last week to make plasma. Poor beaker.
|
|
|
itsallgoodjames
Hazard to Others
  
Posts: 276
Registered: 31-8-2020
Location: America Lite
Member Is Offline
|
|
Quote: Originally posted by JJay  | the thermometer met a tragic end when it was sucked into a flask at high velocity under vacuum
[Edited on 17-7-2018 by JJay] |
This exact thing happened to me yesterday, while vacuum distilling white fuming nitric acid, with a thermometer that had been purchased the day
before. I guess I need to get an electronic thermocouple thermometer thing, because I seem to go through glass thermometers at an astonishing rate...
[Edited on 19-10-2020 by itsallgoodjames]
Nuclear physics is neat. It's a shame it's so regulated...
Now that I think about it, that's probably a good thing. Still annoying though.
|
|
|
Mateo_swe
National Hazard
   
Posts: 548
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
I had 2 filter funnels brake lately when i was cleaning them.
They broke exactly where the porous coarse filter disk is located.
But that was my own fault not being careful enough.
But i have had many things brake in shipping lately.
Filter funnel, quartz glass tubes, anti-splash adapters, lots of stuff.
|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
10 mL bromine spill
Enough to be both unpleasant and annoying.
I keep my bromine in ampoules between 2 and 10 mL. The ampoules are individually wrapped in packing foam and are in a lidded plastic container on a
bed of sodium thiosulfate.
Today while putting some chems away I accidentally knocked the container from the second bottom shelf onto a concrete floor.
As I picked it up I noticed that the plastic lid was bulging. My immediate thought was that there was a leak in one of the ampoules.
It was then that there was a pop sound as the lid came off and there was a cloud of bromine fill the room. I held my breath and was out of the room
within 2 seconds.
I had some splash onto some skin. So jumped into a shower. Skin damage is pretty superficial.
Surveying damage in lab: I sprayed the air with thiosulfate spray I keep at the entry. I have lost my largest ampoule. Some bromine was still in it. I
inverted it into the bed of thiosulfate and popped the lid on. Splashes of bromine were sprinkled with thiosulfate and doused with water. The smell
was uncomfortable enough that I had to leave. It may take a little while for the gas to dissipate. Then I can go back to cleaning up my messes.
Takeaways: I can probably improve my packing and storing but the concept is good. The situation could have been a whole lot worse. Thiosulfate is good
to have around and easy to grab. Easy egress from the lab is a good thing to have. My painted cupboard door may bear the signs of this incident for a
while.
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
I suggest sealing all that stuff in larg-ish ziploc bags. They are made out of polyethene or polypropene and they allow for a lot of expansion when
sealed flat.
I actually seal everything in ziploc bags nowadays, including my glassware. Less noisy to store and less likely to break due to cushioning effect, and
if you seal them a bit inflated, they are self-formed airbags.
|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I don't think ziplocks wouls have been any better than the container I was using (press sealed honey container similar to tupperware.) Both would have
popped in yhe temp/pressure conditions.
If anyone is interested, here is what a bromine splash looks like after 24 hours.
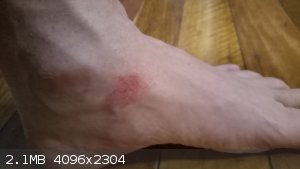
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
You could put neutralizing agent on bottom of the bag. It would consume the reactant gas as it was produced. Of course, with expectation that there
will be no gaseous byproducts.
|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Quote: Originally posted by Fyndium  | | You could put neutralizing agent on bottom of the bag. It would consume the reactant gas as it was produced. Of course, with expectation that there
will be no gaseous byproducts. |
Again, I had a bed of neutralising agent in the plastic container. And I think my plastic container is a bit more robust and has greater longevity
than a zinpock bag.
The difference would come down to the bag being more likely to leak whereas the container had the lid pop off percussively.
The weak point in my system is insufficeint packaging. The tip of the offending ampoule was exposed, being a bit taller than the others. And the
glass was thin there. I had recently used a couple of ampoules and so there was some rattle room in there. The very tip of the ampoule was knocked
off.
Also, I should have backed off when I saw the lid bulging. It was not a good thing to go off in my hand.
That said, it is not the first time that something in my lab has gone wrong but I have been saved by packing things in small quantities and having
neutralising agents right on hand.
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
My cheap $50 Zeny single stage rotary vane vacuum pump is no longer pulling a full vacuum, even after I changed the oil. Im concerned that organic
vapors messed it up somehow
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Online
|
|
Cou - freezing trap cooled with CO2 ice + organic solvent is necessary to insert before the pump... or if someone has access to liquid N2 even
better... maybe at least crushed ice + NaCl (T = -18 C) or crushed ice + CaCl2 ?
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
It's too expensive to buy a block of dry ice for just one vacuum distillation, when the rest will sublime away.
Crushed ice + NaCl could condense water vapor, but not organic solvents such as ethyl acetate. I don't know why those would damage the pump. They
would just mix with the vacuum oil.
EDIT: I just checked the connections and it's probably not pulling full vacuum anymore because the plumbing tape has worn out.
[Edited on 11-1-2020 by Cou]
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
Use calcium chloride to bring down the cooling bath to -35C or whatever your freezer goes. It's not as efficient as fancy dry-ice-acetone-liquid
helium baths, but it doesn't cost a dime either. For most amateurs, it's a fact that dry ice bath is well beyond their economics, unless they're
cooking some high value stuff. As stupid as it sounds, if this is the case, in long run it could be cheaper to stock new vacuum pumps instead of dry
ice. Another "benefit" is the ability to vacuum corrosive stuff.
I'm not sure how this thing works actually, but I know that CaCl2 dissolution is very exothermic, but if one could prepare a concentrated solution of
it pre-cooled at freezer, and add that with solid ice blocks, should this keep the temp low?
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Freezer only goes to -17 c.
[Edited on 11-1-2020 by Cou]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Not chemistry glassware, but the other night, I broke a dragon-claw-themed wine glass that my mother had given me about ten or so years ago (which she
had given me to replace a similar one that she had given me ten or fifteen years prior, after my then-wife broke it). Alas.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
Don't work when tired. I did a post reaction cleanup and drained the washwaters - and accidentally poured half a liter of toluene that was stripped
from the reaction. I realized this only later when I was looking for the flask and saw it was alongside the washwater flasks, all cleaned and shiny. I
had even placed the flask away to not mess it up with the water flasks, but apparently it was not enough.
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Until today I was of the opinion that evaporating basins could take a bit of heat.
This morning I wanted to do a crude test for the percentage of sodium chlorate in a mixture of what I suspect was mostly sodium chloride.
To 1 g of the crystalline mixture I added 0.5 grams of sucrose and applied heat.
Turns out the crystalline mixture was mostly sodium chlorate and the little pile burned brightly for a couple of seconds followed by a cracking noise
as my evaporating dish fell in half  . .
|
|
|
Piroz
Harmless

Posts: 19
Registered: 15-12-2020
Location: Poland
Member Is Offline
|
|
Two weeks ago I tried to make nitric acid by dissolving NO2 in water. NO2 was generated in steel beercans (yes, in my country some beercans are made
of steel, not aluminium  ) by heating anhydrous calcium nitrate in embers of
charcoal. I made same experiment in April and I got liquid N2O4/NO2. Unfortunately this time calcium nitrate wasn't dehydrated properly, nitric acid
formed inside and created hole. Luckily that was cold part of apparatus so I changed it into undamaged beercan and NO2 generated again. Few minutes
later I had the second failure - barbecue blower that I used for glowing charcoal broken down. I decided to glow charcoal by the other way- using
garbage bag and hose (an idea from last experiments), but temperature was too high and one of beercans was overheated. It created bigger hole without
any hope to repair. ) by heating anhydrous calcium nitrate in embers of
charcoal. I made same experiment in April and I got liquid N2O4/NO2. Unfortunately this time calcium nitrate wasn't dehydrated properly, nitric acid
formed inside and created hole. Luckily that was cold part of apparatus so I changed it into undamaged beercan and NO2 generated again. Few minutes
later I had the second failure - barbecue blower that I used for glowing charcoal broken down. I decided to glow charcoal by the other way- using
garbage bag and hose (an idea from last experiments), but temperature was too high and one of beercans was overheated. It created bigger hole without
any hope to repair.
 
[Edited on 23-12-2020 by Piroz]
[Edited on 23-12-2020 by Piroz]

|
|
|
| Pages:
1
..
34
35
36
37
38 |