jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
Compact Flow-Through Electrochemical Cell for Perchlorates
Here's a paper I recently found that may be of interest.
Attachment: TOCENGJ-13-23.pdf (1.6MB)
This file has been downloaded 472 times
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
ive heard of this type of cell before and never really tested it but a bed of powder PbO2 made from anodizing lead in sulfuric acid solution might
work for this and all you need is a graphite rod inserted in that bed in a dry area of it.
Doing so might allow the amateur to make a batch mode process out of this setup.
According to that paper he used an MMO ribbon or tube for corrosion control as the anodic current feed.
MMO will survive a perchlorate cell in its early stages but it makes sense that the bed is at continuous flow which prevents the buildup of
perchlorate which can damage some types of MMO.
recommended to use magnetite made by a furnace at 800 Celsius and carbon steel + steam for your current carrier as while that will absolutely be
inactive as a material for making perchlorate I heard it can survive perchlorate cell conditions.
You can try MMO as the current collector but I think its a bad idea in an amateur setting.
[Edited on 2-10-2020 by mysteriusbhoice]
|
|
|
yobbo II
National Hazard
   
Posts: 762
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
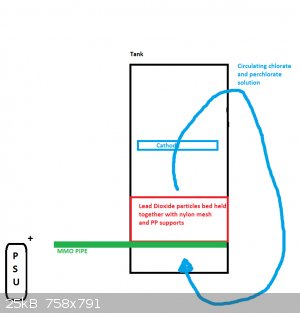
It reminds me of the Pinkston anode.
http://www.pyrosociety.org.uk/forum/topic/426-synthisis-of-p...
A great big bag of lead dioxide pieces with a graphite rod stuck into them for a Perk. anode.
How long will the current feeder last? It's simply an MMO tube (not simple at all). A Ti pipe coated with MMO.
I suppose a mesh will do instead.
Will it not erode in a perchlorate cell.
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
MMO wont erode in a perchlorate cell if its only used as a carrier and not the main electrode but I have my doubts of making this process work in
labscale.
|
|
|
yobbo II
National Hazard
   
Posts: 762
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
It may erode as the current carrier. It will be exposed to the solution and some current will go from the MMO into the solution. ie. it will be
working as an anode whether you want it or not.
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
i remember something about fiber cloth wrapped around graphite electrodes to keep them together, it supposedly worked but never got to try it out
myself... so methinks- same deal could be done with PbO2 maybe? PbO2 decomposes at 290*C apparently, what if someone pressed PbO2 with an inert
material, maybe grinded down the rods well so the surface would be perfectly smooth- i have no idea what materials would work well for pressing, and
even less which would be able to resist electrolytic cell
as for the PDF, i dont quite understand- do they say that titanium metal plates wouldnt work out for squashing the lead dioxide in between? if one
could make loads of tiny little holes, or maybe somehow produce chips of PbO2 and then sandwich that inbetween some titanium mesh that should work
too, yea? it just seems weird to me that the titanium would entirely passivate like that
i really dont fancy the idea of plastic due to its low melting point
maybe if PbO2 was put into a steel pipe and the steel pipe was heated just enough to contract it would give many tonnes of pressure to force the PbO2
into some compacted form
|
|
|