kazaa81
Hazard to Others
  
Posts: 368
Registered: 30-4-2004
Member Is Offline
Mood: ok
|
|
Purification of ethylene glycol from antifreeze
Hello,
I'm searching for a method of purication of ethylene glycol from
antifreeze car liquid.
On the label, it only says "contains ethylene glycol" and "bitter-tasting substances added to prevent drinking".
I've tried to check some data on CRC handbook of chemistry and physics, but it seems there exist so much ethylene glycols.
Thanks for help
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Distill it.
|
|
|
kazaa81
Hazard to Others
  
Posts: 368
Registered: 30-4-2004
Member Is Offline
Mood: ok
|
|
What if it's diluted with substances that distill first of it, like methanol?
The problem I'm worried about is methanol toxicity, rather than just separing ethylene glycol.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
So collect the intermediate distillates. Watch still head temp for when to change.
Tim
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
At as high a temperature as the distillation occurs
it is for sure you won't keep any methanol in the
distillate if you are distilling fairly rapidly with only tap water cooling of the condenser ......any methanol will
blow right through without condensing . What you will get coming over with the glycol is water and a good bit of it maybe 10% or more , because it
is a non listed
ingredient in the antifreeze from when it is watered down , stepped on by the manufacturer in order to rip off the consumer for the water weight .
I am not sure if it will fractionate out or if there is an azeotrope with the glycol , as I didn't watch the temperature during distillation , but
density measurement of the distillate
tells the tale . There is a density table attached to my post in the thread linked above .
|
|
|
Al Koholic
Hazard to Self
 
Posts: 98
Registered: 2-12-2002
Member Is Offline
Mood: Seeking ligand
|
|
Azeotrope
I'm fairly certain there is at least some intermediate azeotrope during this distillation. At the moment I have some running and in all my
separations I notice a very slow climb to the 197C BP of pure glycol.
The T stops around 110 for about 20 minutes with strong heating while mostly water comes over and then it takes another 30 minutes or so to reach 197,
possibly longer, I should time it sometime. During this time distillate is continuously coming over and has a good EG smell to it but is clearly not
pure. I haven't the will right now to collect samples from many different runs at specific T's to do a chart on T v.s. SpGr. Though that may be
interesting....
Anyway, if you start keeping distillate before 197C you are probably not getting pure glycol. Though since it is likely water contamination, it might
not be a big deal if you dry the product anyway.
[Edited on 12-8-2006 by Al Koholic]
|
|
|
boilingstone
Harmless

Posts: 8
Registered: 26-5-2017
Member Is Offline
Mood: No Mood
|
|
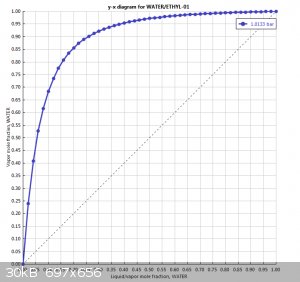
Here is a VLE diagram for water-ethylene glycol. Looks like there shouldn't be any azeotropes here.
|
|
|