Texium
Administrator
       
Posts: 4618
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Synthesis of Hexamethylenetetramine (Hexamine)
First of all, let me get this out of the way: I know that this procedure is Amateur Chemist Heresy, akin to making aspirin using acetic anhydride, but
I need hexamine for a future synthesis and didn't have any. I've been avoiding going out to stores for anything besides groceries, due to the
pandemic, so trying to find fuel tablets was off the table. I happen to have a lot of formaldehyde solution and ammonia on hand, so I just decided to
make it.
Safety Considerations:
I performed this reaction in a fume hood. Formaldehyde is very toxic and a probable carcinogen. It should not be handled without a fume hood, a
comparable source of ventilation, or a respirator rated for handling formaldehyde.
Ammonia is much less scary, but its vapors are still very annoying to deal with. Since it is used in excess, a lot will be evolved when evaporating
the solution. I used my rotovap for this purpose, and passed the vacuum pump effluent through a flask of vinegar to trap most of the ammonia that made
it through. If you boil it off on a hot plate, make sure to do it in a fume hood or other well-ventilated area.
Experimental:
140 g of 37w% aqueous formaldehyde (Sigma-Aldrich) was added to a 500 mL erlenmeyer flask along with a magnetic stir bar.
With moderate stirring, 200 mL of 10% aqueous ammonia (ACE janitorial strength) was added cautiously to the flask in several portions. The temperature
climbed quickly throughout the ammonia addition, peaking at 68ºC shortly after completion.

The reaction was allowed to run overnight. The pH of the solution the next morning was neutral by litmus, so an additional 50 mL of 10% ammonia was
added to ensure completion, and it was allowed to stir for another hour.
The solution was evaporated to yield a white paste.

Recrystallization from water and isopropanol was attempted, but failed to produce crystals, so the solution was evaporated again, and the resulting
damp solid (44.0 g) was dried in a vacuum desiccator.
The mass of the product was checked every hour, holding steady at 37.1 g after 5 hours.

Results/Discussion:
The product is a free-flowing white powder with a faint amine odor. It burns with an orange flame, producing no visible smoke and leaving behind black
residue.
Yield: 37.1 grams (93% based on formaldehyde).
The yield was satisfactory, but likely could be improved by cooling the reaction mixture during the ammonia addition. Some bubbles were evolved from
the reaction mixture as it heated up, which were most likely formaldehyde leaving solution.

|
|
|
morganbw
National Hazard
   
Posts: 561
Registered: 23-11-2014
Member Is Offline
Mood: No Mood
|
|
@Texium (zts16)
Thank you. I firmly believe that many/many of the simple synthesis need to be posted.
|
|
|
Bmoore55
Hazard to Self
 
Posts: 85
Registered: 23-7-2018
Location: Texas
Member Is Offline
|
|
Very nice work! During a pandemic you have to do what you can to get the chems you need. Hit me up if you need a purity check on this product.
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Thank you for posting! I was not familiar with this reaction, but probably should have realised.
I totally agree with you @morganbw!
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Can ammonia solution be more concentrated, or would it affect the yield?
|
|
|
dawt
Hazard to Self
 
Posts: 74
Registered: 9-5-2016
Location: EU
Member Is Offline
Mood: fluorescent
|
|
I'd assume it'd work fine, just make sure you have proper cooling during the addition.
|
|
|
vibbzlab
Hazard to Others
  
Posts: 241
Registered: 6-11-2019
Member Is Offline
Mood: Always curious
|
|
Synthesis of Hexamine / Urotropine
Hexamine or Urotropine is an organic compound used as a urinary antiseptic and more than that its widely used as a camping fuel as it burns pretty
smoothly without producing smoke or leaving behind ash.It is also a precursor of RDX which i dont recommend through this prep matter
If you dont want to keep reading you can directly go to my preparation video ,link below or you can watch that after my writeup
https://youtu.be/K8qOqMosXlU
Materials required.
Overall the preparation is very easy.
We just need
1) Formaldehyde 37% -23.5g
2)Ammonia 25% -45g
Just the right stoichiometry can give you fairly good amount of result.
Just a small note. Chill both the reactants before the experiment and also if u can ( which i didnt) use an ice bath for this experiment.
Procedure
The method of synthesis is also extremely easy.
Just take a big beaker like a 400ml one and pour in the cold formaldehyde into it.
Then pour in cold ammonia solution slowly with strong stirring. The reaction is exothermic and it will heat up. Thats why I recommended to use an ice
bath and chilled reagents, as you know heat can disperse out both the formaldehyde and ammonia gas from the reaction mixture which wont be very cool.
Try doing it in a well ventillated area or in a fume hood.
After adding the ammonia,continue stirring for another 15-30minutes. Then allow the mixture to stay undisturbed for 24hours .Just close the lid of
beaker with a watch glass or something and keep aside and it will be fine.
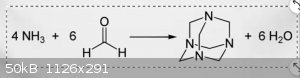
Whats happening is 6molecules each of ammonia and formaldehyde reacts to form hexamine.I will include the reaction diagram in the attachments.
After 24 hours just take a flat plane tray and pour the contents into that and keep it under sun or any other hot windy source to evaporate out all
the water. You can see urotropine crystallising out. I have also included a picture of that in attachments.
You can definetly test the compound by taking a few amount and setting it on fire xD.
So yea thats the preparation as a whole. Hope everyone would like it.
Thank you
vibzzlab
Amateur chemist. Doctor by profession
Have a small cute home chemistry lab.

Please do check out my lab in YouTube link below
This is my YouTube channel |
|
|
vibbzlab
Hazard to Others
  
Posts: 241
Registered: 6-11-2019
Member Is Offline
Mood: Always curious
|
|
well i guess i messed up with the images
I messed up. Its actually 4mol ammonia and 6 mol formaldehyde and this is the reaction
Amateur chemist. Doctor by profession
Have a small cute home chemistry lab.

Please do check out my lab in YouTube link below
This is my YouTube channel |
|
|
Texium
|
Threads Merged
2-6-2020 at 06:21 |
Pumukli
National Hazard
   
Posts: 708
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Texium, it was great!
Do you know what kind of contaminant can cause the yellow (soothy) flame and the black residue? Formaldehyde solution supposedly contain only
formaldehyde, methanol and water (along some polimeric form of formaldehyde). I assume all these compounds would burn fairly well without residual
carbon. Something in the ammonia solution?
|
|
|
Texium
Administrator
       
Posts: 4618
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
I don’t think the flame color is due to contaminants. I’m pretty sure that’s just how hexamine tends to burn in air. It’s a very energy dense
fuel, but with only air present as an oxygen source, I don’t find it surprising for it to burn with a yellow flame, even when pure. Also in spite of
the low temperature flame and residue left on the watch glass, there was not any visible smoke produced when it burned.
|
|
|