vibbzlab
Hazard to Others
  
Posts: 241
Registered: 6-11-2019
Member Is Offline
Mood: Always curious
|
|
Synthesis of Potassium chromate and dichromate from Chromic oxide
Hello friends.
I am just giving a write up of the video i did for preparing Potassium chromate and dichromate from Chromic oxide by oxidisng it.
If you want to directly watch my prep video
https://youtu.be/5gMN6Kc0HGk
otherwise continue reading.
Materials required
6.5g KOH
5g Cr2O3
5g KNO3
chromic oxide is available as the chrome green pigment. I used the leftovers from ammonium dichromate decomposition after washing and drying to remove
any unreacted ammonium dichromate crystals.
Start by heating a metal bowl and transferring the KOH into it.
Continue heating untik KOH melt
Give a gentle mixing so that all the solid chunk melts and it form a uniform liquid
Once its a uniform liquid transfer chromic oxide into it and mix well
When everything has become a thin paste add Potassium nitrate and mix up everything.
Continue heating for 20minutes
What you observe is a redox reaction.
Potassium nitrate us the oxidising agent and Chromic oxide is reducing agent
By 20minutes the mixture has become a yellow paste .Now put off the flame and let it cool.
Transfer it into a beaker and dissolve in water to form a yellow solution. Filter the solution to remove any unreacted debri and there we have the
potassium chromate solution.
Boil off excess water and cool to crystallise out the crystals of potassium chromate.
Now to make potassium dichromate we take the crystals of chromate and make a saturated solution.
Acidify it with some glacial acetic acid.(4ml)
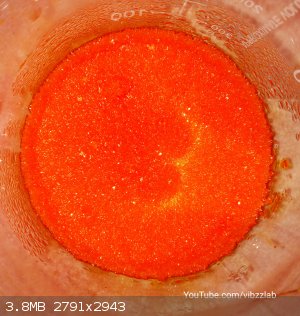
We now have a deep orange solution
Chromate to dichromate conversion is dependent on pH.(Acidic pH favours this side)
As usual boil of excess water and cool to crystallise the crystals of potassium dichromate.
pack them both in airtight sealed container
Now to watch dp check the video,link below
https://youtu.be/5gMN6Kc0HGk
Amateur chemist. Doctor by profession
Have a small cute home chemistry lab.

Please do check out my lab in YouTube link below
This is my YouTube channel |
|
|
sciece nerd
Harmless

Posts: 25
Registered: 27-8-2019
Member Is Offline
|
|
What's the nitrite contamination in that?(Probably not much due to the high solubility of KNO2)
Also, will adding acid or an ammonium salt remove the nitrite without reducing the chromate?(although using ammonium to react with nitrite to form
nitrogen would create another salt that needs to be removed)
I'm not sure if dichromate would oxide nitric oxide in acidic media)
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Nice work vibbzlab! Good looking products too.
|
|
|
NaK
Hazard to Self
 
Posts: 83
Registered: 12-9-2019
Member Is Offline
|
|
cool but there is no reason to waste your KOH. Just reacting KNO3 and Cr2O3 yields potassium dichromate as well without a second acidification step
and without having to worry about nitrite contamination as the nitrogen just escapes as nitrogen oxide gas (do this outside!)
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
KOH is cheaper and easier to buy here than KNO3 and it also serves other purposes. It helps flux the reaction mixture by lowering the Mp and reducing
viscosity so improving the reaction outcome. You can also use NaOH and NaNO3 which are very easy to buy here (UK) and very cheap.
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
Not knowing anything about UK and EU laws I thought the nitrates are still illegal there?
|
|
|
Bedlasky
International Hazard
    
Posts: 1243
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
No, they are legal. It's very easy to obtain them in pure form. I just look at two webs of my favorite suppliers and they sell Ca, Na, K, NH4 and Sr
nitrates.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
You can buy 85% ammonium nitrate OTC. Just dissolve, settle, decant, boil to dryness and dry in an oven.
|
|
|
chemplayer...
Legendary
  
Posts: 191
Registered: 25-4-2016
Location: Away from the secret island
Member Is Offline
Mood: No Mood
|
|
Nice video, very well done!
|
|
|
subskune
Hazard to Self
 
Posts: 71
Registered: 30-4-2017
Member Is Offline
Mood: No Mood
|
|
I did the same synthesis with manganese dioxide instead of potassium nitrate. The MnO2 can be bought in EU countries or is obtained from batteries,
nitrate is very difficult to get in pure form in the EU.
|
|
|