numos
Hazard to Others
  
Posts: 269
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
Original Jack Roberts compounds
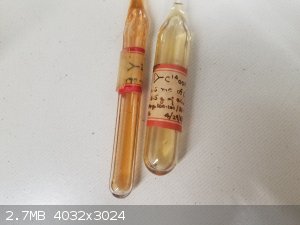
Came across a set of compounds which were made in the lab of Jack Roberts. I've taken pictures of some of the interesting ones - really classic
samples in their original containers and labels. The cyclobutenes especially, are notable as they were used in the development of NMR prediction and
understanding spin systems.
There are many more, but some are decomposed and some don't have all that interesting structures. These are the highlights.
 
 

|
|
|
Dr.Bob
International Hazard
    
Posts: 2761
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: Mildly disgruntled scientist
|
|
Neat find. I always want to save everything like that until I realize I have nowhere to put it all. I have some books from Gertrude Elion's
collection in my books. Sadly, unlike most people, she did not write her name in every book, so they are not very collectable. But still neat to
have a little piece of history. PS, If anyone wants some similar samples, we have about 20 bins of poorly labelled or unknown vials like that from
chemists who retired...
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Did you maybe mean John D. Roberts?
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
Incredibly cool find! Do you know how old they are approximately?
And one of them is apparently labelled with carbon-14! Do you have a geiger counter to check it?
|
|
|
numos
Hazard to Others
  
Posts: 269
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Heptylene  | Incredibly cool find! Do you know how old they are approximately?
And one of them is apparently labelled with carbon-14! Do you have a geiger counter to check it? |
Jack Roberts died just a few years ago so these compounds can't be more than 60-70 years old. In fact I talked to one of his students who worked on
the cyclobutenes as a grad student. He's 90 ish now so those compounds are from ~1950.
Don't have a Geiger counter, but I can ask around here!
Also Amos, Jack was a nickname he preferred to go by, but you are right, his legal name was John.
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
The label on the ampoule with the 14C-labeled compound is marked 4/29/53, which I presume is a date.
BTW, you're unlikely to pick up much if anything with most common Geiger counters. The beta-radiation from 14C is very weak. The glass will block much
of it, and even without glass (but I don't recommend opening that ampoule!), you'll need a probe that is suitable for picking up low-energy beta's.
[Edited on 10-3-2020 by phlogiston]
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by phlogiston  | The label on the ampoule with the 14C-labeled compound is marked 4/29/53, which I presume is a date.
BTW, you're unlikely to pick up much if anything with most common Geiger counters. The beta-radiation from 14C is very weak. The glass will block much
of it, and even without glass (but I don't recommend opening that ampoule!), you'll need a probe that is suitable for picking up low-energy beta's.
[Edited on 10-3-2020 by phlogiston] |
Fair point, but there will be some x-rays from the beta particles stopping in the glass. I think the max. 156 keV betas of 14-C might produce a
response on a typical tube. It's worth a shot.
|
|
|
numos
Hazard to Others
  
Posts: 269
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Heptylene  | Quote: Originally posted by phlogiston  | The label on the ampoule with the 14C-labeled compound is marked 4/29/53, which I presume is a date.
BTW, you're unlikely to pick up much if anything with most common Geiger counters. The beta-radiation from 14C is very weak. The glass will block much
of it, and even without glass (but I don't recommend opening that ampoule!), you'll need a probe that is suitable for picking up low-energy beta's.
Definitely a jump from back
[Edited on 10-3-2020 by phlogiston] |
Fair point, but there will be some x-rays from the beta particles stopping in the glass. I think the max. 156 keV betas of 14-C might produce a
response on a typical tube. It's worth a shot. |
Went ahead and did this. I don't have a Geiger counter but my geologist professor friend had a good one. It was picking up low doses (I think ~0.01
mR/hr but I may be reading this wrong) through the glass. But only through the tube opening, meaning no x-rays/gamma rays. Just a few high energy
betas that made it through the glass. It was definitely a spike from background.
Great idea and neat result - thanks!

|
|
|