| Pages:
1
2
3
..
8 |
lacrima97
Hazard to Self
 
Posts: 93
Registered: 24-7-2005
Location: MS
Member Is Offline
Mood: experimental
|
|
Crystal Growing
I saw something about this a while ago, and I remember my dad helping me grow this blue crystal when I was little, but how does it work? How could
you grow, maybe a CuSO4*H2O for example. How are they actually formed to look so pretty? Is it simply dissolving the substance in a solvent, and
then evaporating the solvent?
|
|
|
kazaa81
Hazard to Others
  
Posts: 368
Registered: 30-4-2004
Member Is Offline
Mood: ok
|
|
Copper Sulphate Pentahydrate
CuSO4 * 5 H2O
CAS #: 7758-99-8
Mol. 249.686 g/mol
Form: blue tricline crystals
M.P. 110°C decomposition
Density 2.286 g/cm^3
Soluble 22.0^25 g in 100ml H2O
Soluble in MeOH, slightly soluble in EtOH
(from CRC press, handbook of chemistry and physics, 85th edition)
Work out of this
|
|
|
DrP
National Hazard
   
Posts: 625
Registered: 28-9-2005
Member Is Offline
Mood: exothermic
|
|
| Quote: |
How are they actually formed to look so pretty? Is it simply dissolving the substance in a solvent, and then evaporating the solvent?
|
This will give you lots of very tiny ones. To get a nice big pretty one you should select the biggest of your small ones to use as a seed. Hang
this seed into a saturated solution of the CuSO4 by a piece of cotton and it will grow! (you can use a lolly stick to hang the cotton from over the
top of a beaker). This may take some practice and some time to get nice big ones... 
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
How do you get it without the string embedded? (What's that do to purity, strictly speaking? LOL)
I've had a few reasonable results with placing a seed crystal in the bottom of a container and evaporating slowly. However, you have to clean it off
periodically as smaller crystals will settle into place and intersect, and any movement (of the solution) or contact with the crystal tends to upset
growth.
Does anyone have a library of crystal habits? That would be as useful as chemical properties in identifying isolated substances.
Tim
|
|
|
lacrima97
Hazard to Self
 
Posts: 93
Registered: 24-7-2005
Location: MS
Member Is Offline
Mood: experimental
|
|
Ah, I see, so just have a crystal for the others to attach to...Hmm, I might give this a try sometime.
|
|
|
bereal511
Hazard to Others
  
Posts: 162
Registered: 9-8-2005
Location: Madison, WI
Member Is Offline
Mood: No Mood
|
|
http://www.waynesthisandthat.com/crystals.htm
I found this site to be quite interesting and helpful in making large, single crystals.
As an adolescent I aspired to lasting fame, I craved factual certainty, and I thirsted for a meaningful vision of human life -- so I became a
scientist. This is like becoming an archbishop so you can meet girls.
-- Matt Cartmill
|
|
|
NeutralIon
Harmless

Posts: 28
Registered: 19-6-2005
Member Is Offline
Mood: No Mood
|
|
I did some crystal growing years ago when I was in high school -- kind of a extra credit chemistry project -- in fact my chem teacher gave me the
chemicals to grow the crystals from.
DrP's comment about hanging a seed crystal on a string is correct -- that will give large, well shaped crystals. Basically you create a saturated
solution in boiling water, then let the solution cool to become super-saturated. Then carefully lower the seed crystal into the solution and let
everything set undisturbed. If it don't work out right press reset -- i.e. just boil again to get everything disolved and start over.
A very good substance to start with is alum, KAl(SO4)2.12H20. This is one of the easier to grow and makes beautiful, clear octahedral crystals.
Chrome alum, KCr(SO4)2.12H20 is another good one -- it makes deep violet octrahedral crystals. I did both of these those many years ago.
For a really neat effect you can even grow a chrome alum crystal and use it as a seed to grow a clear alum crystal over it -- one of the few cases
where a crystal will grow well on a substance other than itself. This leaves you with a perfect violet octahedron inbedded in the center of a clear
one. I never got the chance to try that but I have seen pictures.
Knowledge is Good
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
I too did some crystal growing in my time, by the same method mentioned above. I used potassium sodium tartrate. Attached is a picture of the
crystal which I dug up out of the lab.

|
|
|
MadHatter
International Hazard
    
Posts: 1347
Registered: 9-7-2004
Location: Maine
Member Is Offline
Mood: Enjoying retirement
|
|
Crystal growing
Lacrima97, check your U2U for FTP access. There is an article there by Peter G. Jones that
may help you.
From opening of NCIS New Orleans - It goes a BOOM ! BOOM ! BOOM ! MUHAHAHAHAHAHAHA !
|
|
|
kazaa81
Hazard to Others
  
Posts: 368
Registered: 30-4-2004
Member Is Offline
Mood: ok
|
|
I think that melting the salt and then let it cool slowly is a good method to grow large crystals
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Has to be pure though (impurities prevent the liquid phase from orienting to the crystal, or even nucleate other crystal growth), and done very
carefully, since the melting point is sharp.
With some servos (for crystal pulling) and a temperature feedback system, I *might* be able to do something like that with my induction heater, when I
get it up to power.
Tim
|
|
|
JustMe
Hazard to Others
  
Posts: 111
Registered: 7-8-2003
Member Is Offline
Mood: No Mood
|
|
Neat site, bereal511.
I've done some crystal growing a long time ago. Evaporation technique/cooling technique depending on the substance. Spent six months growing ammonium
alum crystal. Grown Copper Sulphate hydrate, potassium dichromate.
Yes, the trick is to get a seed crystal, and then, for even growth, carefully turning them on a regular schedule so that all sides have equal access
to the solution.
But when I was doing my old science fair project and growing crystals of transitional metal saccharin compounds I did something unusual. Most of the
compounds were soluble in water, but sparingly so (except for copper) in acetone. For example, I would make a solution of Zinc Saccharin in water, add
acetone until it just started to come out of solution, then warm the mixture enough so that it redissolved. Instant saturated solution. On slow
cooling I got some pretty spectacular crystals (meaning up to a quarter inch).
The slow evaporation method worked well for most of the other compounds, but this was the only way I got good crystals for zinc saccharin.
By the way, I've had a salt crystal growing for more than 30 years! I was in Israel 30 years ago and got a sample of dead sea water and put it into a
small jar. I noticed over the years that a crystal formed on the bottom and the volume decreased ever so little... obviously an imperfect seal. So I
carefully shake it to turn it over every few years. Now it is like a ship in a bottle (small neck), but there is still plenty of solution left. I just
checked, it is about an inch square, 1/4 inch thick and the bottle is about half empty (Probably held only about 3 oz when full.) 30+ years I've had
that puppy, tempted to break the bottle to pull it out... nah, just let it go another few years.
|
|
|
hodges
National Hazard
   
Posts: 525
Registered: 17-12-2003
Location: Midwest
Member Is Offline
|
|
Here is another interesting kind of crystal to grow:
http://www.chymist.com/Crystal%20tree.pdf
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
"For a really neat effect you can even grow a chrome alum crystal and use it as a seed to grow a clear alum crystal over it -- one of the few cases
where a crystal will grow well on a substance other than itself. This leaves you with a perfect violet octahedron inbedded in the center of a clear
one. I never got the chance to try that but I have seen pictures. "
I have done it. Not only that but, if you use shallow trays of the solutions you can grow"rings" of crystals. Grow a small Crhome alum xtal, put it in
a tray with staurated aulm and let that grow a layer round the seed. If you only use a shallow layer of solution the crystals grow wider but not a lot
thicker. Then you can do the same thing with a dish of chrome alum and get a purple ring and so on.
You can also mix chrome alum with ordinary alum and get a range of colours from near black through violets and amathyst to white. Great fun as I
recall.
|
|
|
MadHatter
International Hazard
    
Posts: 1347
Registered: 9-7-2004
Location: Maine
Member Is Offline
Mood: Enjoying retirement
|
|
Barium chloride
I made barium chloride with the carbonate and HCl and sat the mixture off to the side.
I had forgotten about because of other projects. The water evaporated in the course
of a month and made some beautiful crystals that looked like glass in the jar.
From opening of NCIS New Orleans - It goes a BOOM ! BOOM ! BOOM ! MUHAHAHAHAHAHAHA !
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I just finished making a batch of Ba(OH)2*8H2O crystals. They are nice looking glassine flakes which I dried to dampness using a Buchner funnel
sealed to a source of purified air. I'm not going to try to get them any drier as I don't have the required dessicant or a vacuum dessicator.
[Edited on 24-3-2006 by Magpie]
[Edited on 24-3-2006 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Ashendale
Harmless

Posts: 47
Registered: 3-4-2005
Location: Estonia
Member Is Offline
Mood: Hydrated
|
|
I also tried to grow crystals, took 55 grams of CuSO4 * 5H2O and 100ml of water, heated until all CuSO4 had dissolved and then let it cool.
In the morning I found beautiful CuSO4 * 5H2O crystals on the bottom óf my beaker 
I'll include some pictures (sorry for the quality)
EbC: --> Attachment reduced to allow inline viewing...
[Edited on 14-9-2006 by chemoleo]

|
|
|
Ashendale
Harmless

Posts: 47
Registered: 3-4-2005
Location: Estonia
Member Is Offline
Mood: Hydrated
|
|
Vol 2
(How do you add multiple files to one post? :S)
[Edited on 26-3-2006 by Ashendale]
EbC: As above.
[Edited on 14-9-2006 by chemoleo]

|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Ok, time to show off a little 
The crystals below are CuSO4 of course, and I have four of them, weighing 1.3 kg each, roughly 20 cm long. They have been growing for 2 years, with
the evaporation method. It was a fair bit of work, because the solution had to be filtered frequently (dust, mold), and topped up, while the growth
vessel had to be cleaned out regularly. I grew them in a 10 l bucket (yes with 10 l of CuSO4 solution), because the smaller cups/pots I started off
with became progressively too small. Currently they aren't growing, put I might pick it up at a later time. The 2nd/3rd crystal are a bit rough on the
right edge, because the last time round it evaporated onto the crystal level, making it all mingy and producing this turquoise amorphous stuff. This
is not a problem though, I will scrape it off with a knife, wash it with dH2O a bit, to regrow it in CuSO4 once more. Another problem was the nylon
thread I used initially - it was too thin and too weak, so the weight eventually ripped it. The current nylon thread takes 9kg, so I still have some
leeway. But if you plan big crystals, use the stronger thread from the start, because fixing it later onto a grown smooth crystal (such as the first
one) is very difficult, in fact I had to saw into edges a little to fixate the thread. Again, no problem if it is cleaned nicely after that, and
regrown. Sadly the quality of the crystal suffered a little from it. However, if you grow it long enough, the crystal layers become so dense you can't
ever see the fault.
Other than that, they are the proudest pieces of my collection!
Mephisto upped those pictures on LambdaSyn, with more details given there (German).
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Darkblade48
Hazard to Others
  
Posts: 411
Registered: 27-3-2005
Location: Canada
Member Is Offline
Mood: No Mood
|
|
 1.3 kg! Each!!!! 1.3 kg! Each!!!!
Those are pretty impressive chemoleo, though I don't think I have any plans for getting a 10 L bucket to grow crystals in just yet 
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Those crystals are truly enormous. I have never seen something like that produced by a non- commercial crystalgrower.
Research has really mastered the technique of crystal growing though... I once saw a picture of a 320kg crystal of potassium dihydrogen phosphate.
They used a special apparatus, with continuous dissolving of fresh material and circulation of the solution, together with an elaborate system of
heaters, coolers and pumps to constantly create a very slightly oversaturated solution in the tank.
Those are normally cut into thin slices and used for lasers, for shifting wavelengths.
With the interest in crystalgrowing here, I just started a small batch of CuSO4. 40g CuSO4 Pentahydrate in 50ml water, heated until dissolution and
left to stand covered overnight. I'll see what has formed in there by tomorrow.
I'm interested in growing crystals with unusual materials.
I'll try to create single rhombic crystals of sulfur, by very slow cooling of a saturated solution of sulfur in hot toluene (the evaporation method is
not appropriate here, because the solubility of sulfur in toluene at room temperature is too low).
Alternatively, I could use CS2 and the evaporation method (though that would have to be done outside due to toxicity).
The evaporation would have to be slowed down by a cover over the beaker, the CS2 would evaporate too fast otherwise.
I also poured some 45% NaClO3 solution (of which I have several liters) into a shallow plastic dish (evaporation method), because I read that NaClO3
can also be used for growing crystals.
A really cool thing would be to hydrothermally grow crystals of quartz.
SiO2 is quite soluble in water at temperatures around 300°C, and therefore crystals can be grown in an autoclave.
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Well thank you. Very interesting what you mention on quartz crystals, I have one sitting right on top of my computer screen, and I always wondered
under what conditions it might have formed. I alwyas thought it was formed by melting and cooling, but never could explain why it would, for instance,
sit in those globular rock balls - which are cut apart and contain lots of quartz crystals. Now I know - who would have thought SiO2 is soluble in
water at 300 deg C ? I gotta get one heavy duty autoclave! Do you have more
information on SiO2 crystals, i.e. how they are made commerically? I gotta get one heavy duty autoclave! Do you have more
information on SiO2 crystals, i.e. how they are made commerically?
As to the KH2PO4 crystals - I actually saved an article on this from the BBC website, published 15th Feb 2000:
| Quote: |
Lab grows monster crystal
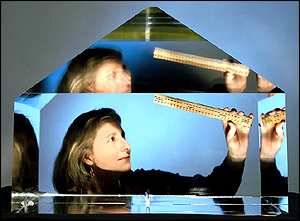
It measures 66 (cm) by 53 by 58.
If you ever struggled to grow copper sulphate crystals in a jam jar at school, look enviously at this monster.
The pyramid-shaped KDP (potassium dihydrogen phosphate) crystal is a record breaker. It weighs in at an astonishing 318 kilograms (701 lbs) - more
than 20 kilos heavier than the previous record holder.
It was grown in just 52 days by researchers at the US Department of Energy's Lawrence Livermore National Laboratory, California.
The enormous crystal is to be sliced into plates for use in a giant laser now under construction at Lawrence Livermore. The crystal plates will
convert the laser's infrared light beams to ultraviolet light just before the beams strike the laser target.
The new equipment will test the safety and reliability of America's nuclear weapons stockpile.

The growth technique builds on Russian methods.
Russian technique
To grow crystals this big, Lawrence Livermore built on a rapid-growth technique first developed in Russia.
A thumbnail-sized seed crystal is placed inside a two-metre-high tank filled with nearly a tonne of supersaturated KDP solution at 65 degrees Celsius
(150 degrees Fahrenheit).
The temperature is gradually decreased to maintain supersaturation as the growing crystal extracts salt from the solution.
The record size of the latest crystal was achieved by giving the solution a transfusion of additional salt through a device called a continuous
filtration system, which helps maintain crystal quality.
"This technique offers the possibility of producing even larger and higher quality crystals in the future," said Ruth Hawley-Fedder, group leader for
the Livermore crystal growing team.
"Our newest record holder could have grown even larger, but we simply ran out of room in our growth tank."
Images by Jackie McBride and Bryan Quintard, Lawrence Livermore National Laboratory |
What I don't quite understand about this technique is how they keep the crystal afloat, there doesn't appear to be a fat nylon cable suspending the
crystal. I almost suspect they just place a nice seed crystal at the bottom of the vat, flat side down, and just let it grow. I don't know the
crystal space group of KH2PO4, but possibly the bottom side of the crystal isn't what the natural crystal would look like.
[Edited on 27-3-2006 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
lacrima97
Hazard to Self
 
Posts: 93
Registered: 24-7-2005
Location: MS
Member Is Offline
Mood: experimental
|
|
Very Very nice chemoleo, thank you for the inspiration, I will most definately be attempting this project myself, 
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Not as big as some natural crystals but very impressive, and the clarity...
NaClO3 IIRC forms crystals that are chiral (there are left and right handed versions).
Crystals of NaNO3 act like iceland spar and split an image in two.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Here is a nice description of the hydrothermal growth process for quartz crystals.
http://www.roditi.com/SingleCrystal/Quartz/Hydrothermal_Grow...
Note how the "Low Pressure Process" uses "only" 700- 1000 bar. 
My attempt at growing large sulfur crystals failed.
At first, only monoclinic crystals were formed, very long and very thin needles.
I read up and it found that at temperatures of about 95°C, only the monoclinic form of sulfur is stable.
So I added more toluene (a total of 65ml for 6g sulfur) to lower the temperature at which sulfur begins to crystallise, and also 1ml of CS2 (I know
that slow evaporation of a CS2 solution of sulfur makes rhombic crystals, so I thought that a small amount of CS2 could favor rhombic shape).
I used a water bath to heat the hermetically sealed vessel, and let it cool down in the covered water bath (the water bath serves as thermal ballast
to slow down the cooling).
It actually makes rhombic crystals now, but they are small and there are a lot of them. They also have this very strange behavior to grow into needles
with the rhombic crystals on them like a string of pearls.
The individual crystals are very good though, a pure yellow and absolutely clear. They also redissolve very slowly, even when the toluene is hot
enough to allow complete dissolution.
The crystalline sulfur would look great as an element sample, much nicer than the powder one always sees.
|
|
|
| Pages:
1
2
3
..
8 |