rockyit98
Hazard to Others
  
Posts: 283
Registered: 12-4-2019
Location: The Known Universe
Member Is Offline
Mood: no mood is a good mood
|
|
source of triethylene glycol for making CROWN ETHER?
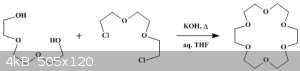
i need triethylene glycol to make 1,2-bis(2-chloroethoxy)ethane and react them together to make
18-CROWN-6 AKA [1,4,7,10,13,16-Hexaoxacycloöctadecane]
using this procedure.
http://www.orgsyn.org/demo.aspx?prep=CV6P0301
if some one know better route please let me know.
|
|
|
Tellurium
Hazard to Self
 
Posts: 84
Registered: 12-7-2017
Location: Group 16, Chalcogen City
Member Is Offline
Mood: smelly
|
|
I would just buy the TEG, as it is very cheap and readily available. But have you found any informations about the 1,2-bis(2-chloroethoxy)ethane?
Synthesis should be easy, but I mean Informations regarding the toxicity? Because "comparable" substances like Bis(chloromethyl)ether are quite toxic
and also highly carcinogenic
[Edited on 16-9-2019 by Tellurium]
|
|
|
rockyit98
Hazard to Others
  
Posts: 283
Registered: 12-4-2019
Location: The Known Universe
Member Is Offline
Mood: no mood is a good mood
|
|
Quote: Originally posted by Tellurium  | I would just buy the TEG, as it is very cheap and readily available. But have you found any informations about the 1,2-bis(2-chloroethoxy)ethane?
Synthesis should be easy, but I mean Informations regarding the toxicity? Because "comparable" substances like Bis(chloromethyl)ether are quite toxic
and also highly carcinogenic
[Edited on 16-9-2019 by Tellurium] |
if they as much as toxic as you say they are perhaps using DEG (Diethylene glycol) to make and bis(chloroethyl) ether and react it with catechol to
make Dibenzo-18-crown-6.
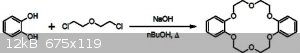
[Edited on 17-9-2019 by rockyit98]
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Bis(chloroethyl) ether is even worse for toxicity. It's basically mustard gas with O instead of S.
I'd expect 1,2-bis(2-chloroethoxy)ethane to be "not great, not terrible." It's got low vapor pressure so I think normal precautions (gloves, lab coat,
fume hood) would be sufficient. I've attached the MSDS from Sigma.
Attachment: msds.pdf (142kB)
This file has been downloaded 295 times
|
|
|
Godrick VanHess
Harmless

Posts: 14
Registered: 24-2-2017
Member Is Offline
Mood: Ionized
|
|
Reminds me of sulfur mustard. Not sure how the O will change its chemical properties but I suspect that it will be similar. I'd be sure to take your
chemical hygiene seriously here.
Reach for the stars, at worst you will go out with a bang.
|
|
|