| Pages:
1
..
63
64
65
66
67
..
77 |
Bedlasky
International Hazard
    
Posts: 1242
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Hi.
There is few photos of my crystals. The blue crystals are CuSO4.5H2O - the bigger one grew one year, maybe longer. They grew up on copper wire - it
wasn't the best option, but they were my first crystals. First was gift for friend and second will be for my girlfriend  . .
The violet one in transparent crystal is K(Al,Cr)(SO4)2.12H2O. It is coated by potassium alum. I made it by mixing potassium alum and chrome alum in
ratio 10:1. Another gift for my friend.
The pale violet crystals are NH4Fe(SO4)2.12H2O. This two crystals are growing really fast - they are one day old and still growing in solution.
The pale violet and rusty crystals in bowl are also ammonium-ferric sulfate. After I pulled out these crystals out of solution, they were rusty
(because of colour of solution) but after a some time were pale violet. Later their colour disappeared and after few hours they were rusty. This
compound really quickly loses water.
The green crystals are CuCl2.2H2O. They are currently turquoise.
     
  
|
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
the potassium-chromium crystal in a crystal is really cool
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Bedlasky
International Hazard
    
Posts: 1242
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Ubya: Thanks  . .
Ammonium-ferric sulfate growing up very fast! I just love these crystals  . Here
is few photos from this morning. . Here
is few photos from this morning.
 
[Edited on 9-5-2019 by Bedlasky]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4356
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Crude cinnamic acid, basically solidified blobs of acid mixed with cinnamaldehyde, with crystals of pure acid growing from them.

Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
j_sum1
Administrator
       
Posts: 6334
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Running some tests on an otc product to figure out what might work for an extraction.

|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
@jsum so what exactly are you extracting? And why are some yellow?
[Edited on 190526 by fusso]
|
|
|
j_sum1
Administrator
       
Posts: 6334
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I am being deliberately vague because I think I have found something cool and I would love to solve it myself. It is an otc product based on an ionic
liquid with a somewhat oily consistency and unknown other ingredients. My tests show that it forms emulsions easily.
|
|
|
Phthaloblue
Harmless

Posts: 2
Registered: 26-5-2019
Member Is Offline
|
|
Some nice FeSO4 crystals from a few years ago, made from steel wool and drain cleaner sulfuric acid as per this link. Alas, they're all brown and spoiled now. Picture has been edited to improve quality (brightness/contrast).
Eventually I should save them by redissolving in dilute sulfuric acid, filtering off the crud, reducing the Fe3+ with a little extra iron,
and making some Mohr's salt ((NH4)2Fe(SO4)2·6H2O)

[Edited on 5/26/2019 by Phthaloblue]
|
|
|
oberkarteufel
Harmless

Posts: 47
Registered: 11-12-2018
Member Is Offline
Mood: Mayonesium sulfate
|
|
CoCl2 + isopropanol + water

|
|
|
Mabus
Wiki Master
  
Posts: 238
Registered: 3-11-2013
Member Is Offline
Mood: Energetic
|
|
Quote: Originally posted by Phthaloblue  | Some nice FeSO4 crystals from a few years ago, made from steel wool and drain cleaner sulfuric acid as per this link. Alas, they're all brown and spoiled now. Picture has been edited to improve quality (brightness/contrast).
Eventually I should save them by redissolving in dilute sulfuric acid, filtering off the crud, reducing the Fe3+ with a little extra iron,
and making some Mohr's salt ((NH4)2Fe(SO4)2·6H2O) |
When I recrystallized my FeSO4x7H2O, I washed the crystals with some isopropanol then left them to dry. Even after 6 months they still haven't turned
brown.
|
|
|
Abromination
Hazard to Others
  
Posts: 432
Registered: 10-7-2018
Location: Alaska
Member Is Offline
Mood: 1,4 tar
|
|
No mercury present, but the thought is beautiful.
This will make a nice addition to my element collection.

List of materials made by ScienceMadness.org users:
https://docs.google.com/spreadsheets/d/1nmJ8uq-h4IkXPxD5svnT...
--------------------------------
Elements Collected: H, Li, B, C, N, O, Mg, Al, Si, P, S, Fe, Ni, Cu, Zn, Ag, I, Au, Pb, Bi, Am
Last Acquired: B
Next: Na
-------------- |
|
|
Σldritch
Hazard to Others
  
Posts: 310
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
A failed attempt at Copper Peroxide yielded this
I am at a loss how to make the synthesis work in water. It hates being filtered and decomposes really quick. Make it with too concentrated peroxide
and it goes whoosh. At least it looks pretty this way. Some of the desired product can be seen on the glass rod where it escaped the water.

[Edited on 7-8-2019 by Σldritch]
|
|
|
wg48temp9
National Hazard
   
Posts: 786
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Ice spikes
One frosty morning recently I spotted some ice spikes that had grown out of some water that had frozen in an up turned plastic access cover in my
garden. Previously I had only seen pics of them.

I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 827
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
I second that - please provide your procedure! As far as NaBH4 and HCHO are concerned, they'll just make carbolines with your substrate, so i hope
you've done careful analysis. I just recently learned that the claimed product is itself neurotoxic as well.
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
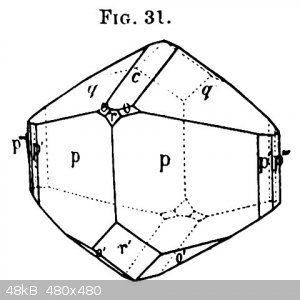
CsCu(SO4)2.6H2O, a Tutton salt

The large crystal is 2cm across and was grown in 2 days. I think that's much too fast to obtain fully transparent crystals.
Tutton himself described the colour as greenish blue. I can't photograph that, but under incandescent light the colour is indeed greenish blue. The
picture was taken under a fluorescent lamp light.
The crystal habit Tutton describes in his 1893 article is in the drawing (Fig. 31).
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Cs2Cu(SO4)2.6H2O of course
|
|
|
Tacticalnuke101
Harmless

Posts: 4
Registered: 12-11-2019
Location: Whitby, Ontario, Canada
Member Is Offline
|
|
Some Tetraamminecopper(II) sulphate complex I made

|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
The two extremes of Cobalt Violet, a cobalt(II) phosphate pigment. The pink one was prepared by adding aqueous ammonia to excess pink(relatively
dilute) CoCl2 and NaH2PO4 solution, while the deep purple one was prepared by adding solid KOH to a boiling hot solution of the two that had been
saturated with salt.

|
|
|
arkoma
Redneck Overlord
      
Posts: 1763
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
Pretty tasty stuff and a pretty picture! Breakfast in San Antonio Tlayacapan, Jalisco

"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Quote: Originally posted by Amos  | | The two extremes of Cobalt Violet, a cobalt(II) phosphate pigment. The pink one was prepared by adding aqueous ammonia to excess pink(relatively
dilute) CoCl2 and NaH2PO4 solution, while the deep purple one was prepared by adding solid KOH to a boiling hot solution of the two that had been
saturated with salt. |
Great colours!
And do you know the composition of both compounds, their formula?
[Edited on 3-3-2020 by Bezaleel]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4356
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
An attempt at K2Cu(malonate)2. Nice xtals, but they turned much paler blue after drying.

Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
anti
Harmless

Posts: 32
Registered: 17-2-2020
Member Is Offline
Mood: Mood: Mood:
|
|
[Edited on 10-3-2020 by anti]
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
Cant u shrink the imgs? theyre unnessarily big and is annoying to scroll and watch.
|
|
|
anti
Harmless

Posts: 32
Registered: 17-2-2020
Member Is Offline
Mood: Mood: Mood:
|
|
Ok
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
And maybe get rid of the non chemistry pictures ?
(I dont want to imagine what they were before shrinking, they're still too big)
The spirit of adventure was upon me. Having nitric acid and copper, I had only to learn what the words 'act upon' meant. - Ira Remsen
|
|
|
| Pages:
1
..
63
64
65
66
67
..
77 |