Jackson
Hazard to Others
  
Posts: 189
Registered: 22-5-2018
Location: U S of A
Member Is Offline
Mood:  Happy about new glassware 
|
|
MeHQ from benzoquinone
Hi, I am looking to synthesize 4-Methoxyphenol from benzoquinone. My question is, Can hydroquinone be replaced with benzoquinone in the reaction? The
reaction would be an acidic benzoquinone solution in methanol reacting in a free radical reaction. Would this work at all? I would be able to get
hydroquinone, but seeing as I already have benzoquinone, it would be preferable.
|
|
|
Schleimsäure
Hazard to Others
  
Posts: 156
Registered: 31-8-2014
Location: good ole Germany
Member Is Offline
Mood: Probably
|
|
In wikipedia it is stated:
4-Methoxyphenol is produced from p-benzoquinone and methanol via a free radical reaction.
However the given sources on that statement only show a start from hydroquinone. In one of these routes p-benzoquinone is used as a catalyst.
See also here:
http://www.sciencemadness.org/talk/viewthread.php?tid=9835
So, my guess as a layman, is no. The double bonds of the oxygens are quite different than the OH-groups of the dihydroxybenzenes.
Somebody more knowledgable than me probably can explain the mechanism.
|
|
|
Jackson
Hazard to Others
  
Posts: 189
Registered: 22-5-2018
Location: U S of A
Member Is Offline
Mood:  Happy about new glassware 
|
|
Yeah, in that thread, the reaction mechanism looks like benzoquinone is the actual thing undergoing the reaction, and it is needed to start the
reaction, because the reaction converts hydroquinone to benzoquinone.
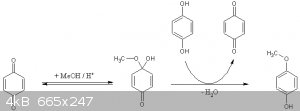
PS: could other alcohols be used for this purpose like ethanol to get 4-ethoxy phenol instead?
[Edited on 2/20/2019 by Jackson]
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Quote: Originally posted by Jackson  |
PS: could other alcohols be used for this purpose like ethanol to get 4-ethoxy phenol instead?
[Edited on 2/20/2019 by Jackson] |
Probably
|
|
|
Jackson
Hazard to Others
  
Posts: 189
Registered: 22-5-2018
Location: U S of A
Member Is Offline
Mood:  Happy about new glassware 
|
|
Okay, guess I’ll just have to try it and report back
|
|
|
Jackson
Hazard to Others
  
Posts: 189
Registered: 22-5-2018
Location: U S of A
Member Is Offline
Mood:  Happy about new glassware 
|
|
Okay, I have tried it with isopropanol and Benzoquinone.
I added about a gram of Benzoquinone to a beaker and then added 91% percent isopropanol until it all dissolved. Then I added about five ml of 96%
sulfuric acid. This was mixed for 24 hours. It was ten neutralized with sodium bicarbonate, which yielded a white precipitate. This was filtered out,
and an attempted recrystalization from naphtha was attempted. It didn’t really dissolve so I tried heating. It didn’t dissolve but it did have a
melting point within a couple degrees of the theoretical melting point. I think it might be the sodium salt of 4-isopropoxyphenol. I put it into the
freezer and it froze into a white chunk on the bottom of the flask. I took it out and put it onto some filter paper to dry.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Jackson  | | It was ten neutralized with sodium bicarbonate, which yielded a white precipitate. This was filtered out, and an attempted recrystalization from
naphtha was attempted. It didn’t really dissolve so I tried heating. |
Sodium sulfate decahydrate is not soluble in naphtha or similar organic solvents.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|