| Pages:
1
..
61
62
63
64
65
..
77 |
VSEPR_VOID
National Hazard
   
Posts: 719
Registered: 1-9-2017
Member Is Offline
Mood: Fullerenes
|
|
Nice iodine, but if you really like halogens you would have posted bromine
Within cells interlinked
Within cells interlinked
Within cells interlinked
|
|
|
Gooferking Science
Hazard to Self
 
Posts: 97
Registered: 17-7-2013
Location: Somewhere in Kansas, USA...
Member Is Offline
Mood: Halogenated
|
|
Here you go:

|
|
|
VSEPR_VOID
National Hazard
   
Posts: 719
Registered: 1-9-2017
Member Is Offline
Mood: Fullerenes
|
|
oi u cheekie wanker, do u got a licence fo that m8?
Within cells interlinked
Within cells interlinked
Within cells interlinked
|
|
|
Chemist007
Harmless

Posts: 3
Registered: 26-10-2018
Member Is Offline
|
|
Awesome...
|
|
|
CarlSagans_RayGuns
Harmless

Posts: 14
Registered: 9-4-2018
Member Is Offline
Mood: No Mood
|
|
Nickel Sulfate crystals
These are some nickel sulfate crystals I made using Nimh batteries.

|
|
|
Jackson
Hazard to Others
  
Posts: 189
Registered: 22-5-2018
Location: U S of A
Member Is Offline
Mood:  Happy about new glassware 
|
|
Copper sulfate that crystalized onto some nickels that i was trying to get nickel sulfate out of.
Also quick question: does nickel sulfate form co-crystals with copper sukfate?

|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
Quote: Originally posted by Jackson  | Copper sulfate that crystalized onto some nickels that i was trying to get nickel sulfate out of.
Also quick question: does nickel sulfate form co-crystals with copper sukfate? |
Sad, you contaminated the
crystals with dirt and oils on your hand...
|
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
Quote: Originally posted by fusso  | Quote: Originally posted by Jackson  | Copper sulfate that crystalized onto some nickels that i was trying to get nickel sulfate out of.
Also quick question: does nickel sulfate form co-crystals with copper sukfate? |
Sad, you contaminated the
crystals with dirt and oils on your hand... |
i don't think that he's going to use them as seeds so i don't see the problem, and even if it was, a quick wash with acetone will remove any oils
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Jackson
Hazard to Others
  
Posts: 189
Registered: 22-5-2018
Location: U S of A
Member Is Offline
Mood:  Happy about new glassware 
|
|
Should i cover them with a varnish if i want to use them as a display piece and preserve them? I assume i should but im not sure.
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
Quote: Originally posted by Jackson  | | Should i cover them with a varnish if i want to use them as a display piece and preserve them? I assume i should but im not sure.
|
Maybe redissolve in water to remove the nickel (coin) and recrystallize to make new crystals?
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Jackson  | | Should i cover them with a varnish if i want to use them as a display piece and preserve them? I assume i should but im not sure.
|
Yes, otherwise they start to lose water and get chalky. I used clear nail polish for mine.
|
|
|
Abromination
Hazard to Others
  
Posts: 432
Registered: 10-7-2018
Location: Alaska
Member Is Offline
Mood: 1,4 tar
|
|
I mostly made this on accident trying to recover a partially decomposed compound.
The compound was iron (iii) salicylate which acts strange under acidic and basic conditions. It seems to me the reactions between it, acids and bases
is in equilibrium because it is all reversible. First, I added acid to give it a clear, yellow appearance and then base to turn it back to its
original purple color. I added slightly to much base, however and a green solution and brownish precipitate formed, as well as some purple. Nothing
had been mixed, so it formed 3 layers of different colors and was absolutely beautiful.

List of materials made by ScienceMadness.org users:
https://docs.google.com/spreadsheets/d/1nmJ8uq-h4IkXPxD5svnT...
--------------------------------
Elements Collected: H, Li, B, C, N, O, Mg, Al, Si, P, S, Fe, Ni, Cu, Zn, Ag, I, Au, Pb, Bi, Am
Last Acquired: B
Next: Na
-------------- |
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
I've tried this before, from what I could tell - no. I've only ever seen one person report a two-transition metal sulfate co-crystal, but I've never
been able to relocate that reference, so I'm dubious.
|
|
|
Abromination
Hazard to Others
  
Posts: 432
Registered: 10-7-2018
Location: Alaska
Member Is Offline
Mood: 1,4 tar
|
|
Iodine I made for my element collection: My first halogen!
I'm going strong with my collection, I am working on synthesizing boron this weekend.

List of materials made by ScienceMadness.org users:
https://docs.google.com/spreadsheets/d/1nmJ8uq-h4IkXPxD5svnT...
--------------------------------
Elements Collected: H, Li, B, C, N, O, Mg, Al, Si, P, S, Fe, Ni, Cu, Zn, Ag, I, Au, Pb, Bi, Am
Last Acquired: B
Next: Na
-------------- |
|
|
j_sum1
Administrator
       
Posts: 6334
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Nice iodine, Abromination.
Before you do boron, make sure you ckeck out MrHomeScientist's videos on it. Both the old and the new. Old is good for procedure. New also mentions
yield and purity issues (significant).
|
|
|
Abromination
Hazard to Others
  
Posts: 432
Registered: 10-7-2018
Location: Alaska
Member Is Offline
Mood: 1,4 tar
|
|
Quote: Originally posted by j_sum1  | Nice iodine, Abromination.
Before you do boron, make sure you ckeck out MrHomeScientist's videos on it. Both the old and the new. Old is good for procedure. New also mentions
yield and purity issues (significant). |
I've seen the new ones, I am pretty much doing exactly what he did except in an iron crucible and am using larger amounts of the oxide.
I'm not entirely sure I have seen the old one, I will go check that out.
List of materials made by ScienceMadness.org users:
https://docs.google.com/spreadsheets/d/1nmJ8uq-h4IkXPxD5svnT...
--------------------------------
Elements Collected: H, Li, B, C, N, O, Mg, Al, Si, P, S, Fe, Ni, Cu, Zn, Ag, I, Au, Pb, Bi, Am
Last Acquired: B
Next: Na
-------------- |
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
 Good luck! I'd love to see the results. Good luck! I'd love to see the results.
|
|
|
Abromination
Hazard to Others
  
Posts: 432
Registered: 10-7-2018
Location: Alaska
Member Is Offline
Mood: 1,4 tar
|
|
We shall see, my magnesium came in today.
List of materials made by ScienceMadness.org users:
https://docs.google.com/spreadsheets/d/1nmJ8uq-h4IkXPxD5svnT...
--------------------------------
Elements Collected: H, Li, B, C, N, O, Mg, Al, Si, P, S, Fe, Ni, Cu, Zn, Ag, I, Au, Pb, Bi, Am
Last Acquired: B
Next: Na
-------------- |
|
|
Abromination
Hazard to Others
  
Posts: 432
Registered: 10-7-2018
Location: Alaska
Member Is Offline
Mood: 1,4 tar
|
|
Ha, well I am going to need a coffee grinder for my boric oxide. Its just too hard for my mortar and pestle. How would you describe the smell of
diborane? Is it almost ozone like?
[Edited on 1-21-19 by Abromination]
List of materials made by ScienceMadness.org users:
https://docs.google.com/spreadsheets/d/1nmJ8uq-h4IkXPxD5svnT...
--------------------------------
Elements Collected: H, Li, B, C, N, O, Mg, Al, Si, P, S, Fe, Ni, Cu, Zn, Ag, I, Au, Pb, Bi, Am
Last Acquired: B
Next: Na
-------------- |
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|

source:
https://twitter.com/elysetwilliams/status/102774533204456243...
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
RogueRose
International Hazard
    
Posts: 1595
Registered: 16-6-2014
Member Is Offline
|
|
I'm not sure how pretty this is, but it is interesting IMO. This is a saturated solution of iron acetate. It had about 2" of solid in the bottom
and about 2" of liquid above it and it was allowed to sit out for about 2 months. About 2 weeks in I noticed a little "puff" on the lip of the bottle
and it steadily grew. I'm guessing that as the water evaporated the crystals grew up the side of the bottle and wicked the other solution. It grew
faster and faster, I'm guessing b/c there was more surface area for the solution to evaporate.
This puff ball was very light, probably weighing about 7-8 grams, which is very little considering the size.
On another note I've found something odd when making the acetate. I used vinegar and allowed nails to sit in a bottle exposed to the air.
Rust/acetate would form above the surface on the nails sticking out of the vinegar and it would be brown/orange. If I added some H2O2 I would get a
nice pink/purpleish solution like a mix of maganese sulfate and KMnO4. Can anyone explain why I would get this color temporiarily as I've never seen
any iron compounds those colors except for some very odd oxides (specially made pigments).
   
|
|
|
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|
Calcium oxalate crystals inside plant cells.
  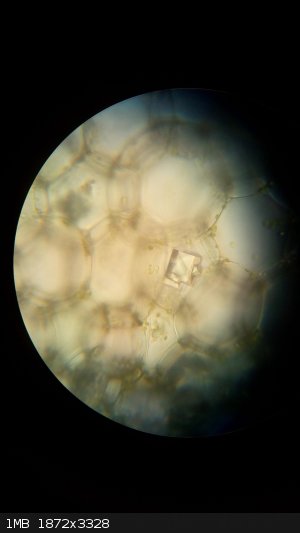
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
So that means plants also get "stones" right?
|
|
|
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|
Well, yeah kind of. The primary reason for the formation of these crystals (also called raphides) is regulation of Ca2+ levels insinde plant tissues.
Some plants (e.g. Dieffenbachia) developed a secondary function and uses needle-like crystals of CaOx as a way to keep animals from eating them (they
cause swelling when ingested).
[Edited on 22-2-2019 by crystal grower]
|
|
|
Pok
potassium Prometheus
  
Posts: 176
Registered: 5-12-2010
Member Is Offline
|
|
Liquid acetylene:

How I made it: video.
|
|
|
| Pages:
1
..
61
62
63
64
65
..
77 |